Abstract
We studied plasma adrenaline (A) in relation to physical fitness, metabolic cardiovascular risk factors and cardiovascular responses. Men (age 21–24 years) with high and normal (both n = 19) screening blood pressure (BP) were studied cross‐sectionally. We measured peak oxygen uptake (VO2peak) (treadmill exercise), and plasma catecholamines, heart rate (HR), finger systolic (SBP) and diastolic (DBP) BP, and insulin‐adjusted glucose disposal rate (GDR/I) during a hyperinsulinaemic glucose clamp (rest) and mental arithmetic stress test (MST). By multiple regression, A at rest (Arest) (β = 0.37, p<0.05) and during MST (Amst) (β = 0.40, p<0.01) were associated with high screening BP. In the respective models, Arest was negatively related to body mass index (BMI) (β = −0.56, p<0.001) and Amst positively to VO2peak (β = 0.54, p<0.001). BP and HR responses correlated positively with VO2peak, but were determined by Amst in multiple regression models. Independently of BMI and VO2peak, serum high‐density lipoprotein cholesterol was positively related to A levels, whereas GDR/I was independently related only to VO2peak. Increased adrenaline secretion may be related to high BP, but may at the same time be associated with a beneficial metabolic profile.
Introduction
Circulating adrenaline (A) is mainly derived from the adrenal medulla Citation[1]. The release of A during stress is thought to improve mental and physical performance, and to be critical for the “fight‐or‐flight” response Citation[2]. Plasma A correlates with blood pressure (BP) Citation[3] and it is often increased in hypertension Citation[4], apparently due to increased secretion Citation[5], but its pathophysiological role is unclear Citation[6]. Mental stress also increases plasma noradrenaline (NA) in young men Citation[7–11], reflecting sympathetic neural activation Citation[1]. According to the “adrenaline hypothesis” Citation[12], A contributes to hypertension by increasing sympathetic transmission, and evidence suggests a link between stress, adrenal medullary activation and high BP Citation[4]. We have repeatedly found correlations between plasma A and BP responses to mental stress in young men Citation[7], Citation[8], Citation[11]. This may be of interest, since increased cardiovascular reactivity may be related to the development of hypertension Citation[13].
Hypertension, obesity and insulin resistance frequently co‐exist, and they are all associated with increased sympathetic activity Citation[14]. The latter partly explains why obesity predisposes to hypertension Citation[15]. The risk of hypertension is also increased by low physical activity Citation[16] and poor fitness Citation[17], regardless of body mass index (BMI). However, increased fitness per se may not reduce sympathetic nerve activity Citation[18], and the reduction of resting heart rate (HR) with exercise training is mainly due to increased vagal cardiac control Citation[19]. Adrenal medullary activity may in fact increase with higher physical fitness Citation[20]. It is important to note that sympathetic neural and adrenal medullary activities can be affected independently by distinct stimuli Citation[2], Citation[21]. Thus, in contrast to sympathetic neural activity, A secretion apparently decreases with increasing obesity Citation[3], Citation[10], Citation[22–28] and is negatively associated with features of the metabolic syndrome Citation[14], Citation[27], Citation[28]. Accordingly, and based on known adrenergic effects on metabolism, Reaven et al. Citation[14] postulated low adrenal medullary activity as a contributor to the lipid abnormalities associated with insulin resistance.
Increased cardiovascular reactivity may be associated with poor fitness Citation[29], but this is not a consistent finding Citation[30]. Similarly, fitness is not consistently related to A responses induced by mental stress Citation[30], Citation[31]. Already published data Citation[11] from the present groups of young men showed significant correlations between BP and plasma A responses to mental stress. When analysing pooled data from several subsets of young men, including the present groups, we found a negative relationship between BMI and plasma A Citation[10]. However, physical fitness may have been a confounder, since higher BMI is consistently associated with lower physical activity Citation[32]. We have shown that fitness is related to insulin sensitivity and vagal cardiac control, assessed from short‐term HR variability Citation[33]. However, we have not addressed potential relationships between A levels and physical fitness or metabolic factors, or the relationship between fitness and cardiovascular responses, in these groups. Therefore, we aimed to compare A levels and responses in men with high and normal screening BP in multivariate models with adjustment for fitness. Based on the background summarized above, we hypothesized that the adrenal medullary response to mental stress is positively related to fitness. With the same reasoning, we also hypothesized that higher plasma A levels are associated with a beneficial lipid profile and insulin sensitivity. Finally, we assessed the relationships of fitness and plasma A with the plasma NA, HR and BP responses.
Methods
Participants
As described previously Citation[11], Citation[33], we studied men born in 1977–1978 who had systolic (SBP)⩾140 mmHg and diastolic BP (DBP)⩾90 mmHg (n = 20) or SBP⩽115 mmHg and DBP⩽75 mmHg (n = 21) at the Oslo military draft examination in 1996. The present data were obtained from the 19 subjects in each group who successfully completed a treadmill exercise test. They were healthy, used no regular medication, and fasted and refrained from smoking for the preceding 10 h and abstained from alcohol for the preceding 24 h. Examinations were conducted at constant room temperature in a quiet room. There were six and seven smokers among men with normal and high screening BP, respectively. In the respective groups, 11 and 10 subjects were weekly engaged in aerobic exercise (average 4 h/week), but this was not significantly related to fitness Citation[33]. As described previously Citation[11], we randomized nine additional men with high screening BP (BMI 24 kg/m2, BP 129/79 mmHg at examination) to a saline infusion study (time control group). We obtained informed consent from each participant and approval by the regional medical research ethics committee. Sitting BP, waist circumference and BMI were measured as described previously Citation[33] and are given in . Correlations between fitness and insulin sensitivity Citation[33] have been published, as have the catecholamine and BP responses to mental stress for the complete groups Citation[11].
Table I. Group characteristics.
Hyperinsulinaemic isoglycaemic glucose clamp and saline control infusion
We applied a 90‐min hyperinsulinaemic isoglycaemic glucose clamp Citation[33]. After baseline blood sampling, insulin infusion (0.86 mU/min/kg body weight) was started and blood glucose was clamped at the fasting level by glucose infusion (200 mg/ml). Insulin sensitivity was expressed as glucose disposal rate (GDR) (final 20‐min glucose infusion rate/weight) divided by serum insulin at 90 min (GDR/I)×100 (arbitrary units) Citation[8]. Clamp infusions were substituted with matched volumes of 0.9% saline in the blinded time control group.
Mental arithmetic stress test
The hyperinsulinaemic glucose clamp does not affect BP, HR or catecholamine responses to the mental arithmetic stress test (MST) Citation[9]. Thus, for practical reasons, MST was carried out immediately after completion of the 90 min required for the glucose clamp, but with infusions maintained constant and participants supine. The participants were first briefly informed of the test. After 2 min of anticipation, they were then instructed to verbally subtract 13 serially from 1079 for 5 min. A metronome was used for distraction, and miscalculations were mentioned. The total stress period thus lasted for 7 min, and was followed by a 15‐min recovery period.
Cardiovascular measurements
R‐R intervals were measured with a Medilog FD4 ECG recorder (Oxford Instruments, Oxon, UK), as described previously Citation[11]. HR was calculated from mean R‐R intervals during the entire pre‐stress period (30 min at baseline before infusions and the glucose clamp), and during MST and the recovery period. SBP and DBP were recorded on the right third finger with a finger BP monitor (Finapres, Ohmeda 2300, Englewood, CO, USA), as described previously Citation[11]. We obtained 15‐min recordings at baseline and during the first, second and third 30‐min period of the clamp, and during MST. The Finapres allows reliable assessment of short‐term BP changes, but may be less accurate for absolute levels Citation[34]. Thus, only BP changes (Δ) are presented.
Exercise test
Fitness was assessed as peak oxygen uptake (VO2peak) during treadmill (Jaeger LE 300, Jaeger, Höchberg, Germany) exercise by a continuous incremental protocol, as described previously Citation[33]. After 4 min of walking at 2.7 km/h, the treadmill was elevated by 1% and the speed increased by 0.6 km/h every 2 min until exhaustion. Respiratory gas exchange was measured breath‐by‐breath using an external volume sensor and the Oxycon Champion gas analyser (Jaeger, Höchberg, Germany). Peak gas exchange and HR values were defined as the highest 30‐s averages obtained. The test was considered satisfactory if respiratory exchange ratio⩾1.05 and HR⩾90% of age‐predicted maximum (208−0.7×age) Citation[35].
Blood sampling and biochemical measurements
Baseline blood samples were drawn through an indwelling i.v. catheter after 45 min of rest. Further samples for catecholamines were drawn after 30, 60 and 90 min during the glucose clamp, within 1 min after announcement of MST, during minutes 1, 2 and 3 of the task, and the last minute of recovery. Serum glucose, triglyceride, total cholesterol and high‐density lipoprotein cholesterol (HDL) concentrations were measured with a Cobas Integra (Roche, Basel, Switzerland), blood glucose during clamp with an Accutrend® sensor (Boehringer Mannheim, USA), serum insulin with an enzyme‐linked two‐site immunoassay (DAKO Diagnostics Ltd., Cambridgeshire, UK), and plasma catecholamines with a radioenzymatic method Citation[36], as described previously Citation[37].
Statistical methods
We used SPSS 11.0 (SPSS Inc., Chicago, IL, USA). Catecholamine levels before (Arest and NArest) and during MST (Amst and NAmst) were averaged over the 4 samples drawn in each situation. We also noted the baseline values, individual maximal A during MST (Amax), and recovery values. Changes (Δ) in all variables were calculated as the difference between mean values at rest and during MST. Non‐parametric tests were used unless normal distribution was achieved by natural logarithmic (ln) transformation. Groups were compared with Student's t‐test or the Mann–Whitney test. Stress responses were assessed by repeated measures analysis of variance. We used Pearson's (r), or when appropriate, Spearman's (rs) correlation coefficients, and standardized regression coefficients (β) from forward stepwise multiple regression models, all adjusted for high screening BP (0 or 1), BMI, waist circumference, smoking status (0 or 1), and VO2peak. Null hypotheses were rejected if p<0.05. Data are given as mean (SD).
Results
Adrenaline, fitness and measures of obesity
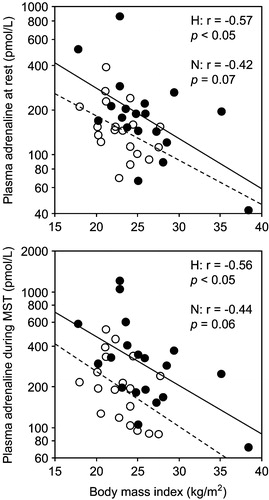
A levels did not differ significantly between the groups (). A levels and ΔA correlated positively with VO2peak in the combined groups () and within the high screening BP group (). Correlations of BMI (r = −0.59, p<0.001) and waist circumference (r = −0.62, p<0.0001) with VO2peak were paralleled by negative correlations with A levels (). BMI correlated significantly with A only in the high screening BP group (), but when the two men without satisfactory exercise tests were included, correlations with Arest (r = −0.55, p<0.05) and Amst (r = −0.54, p<0.05) were also significant in the normal group. As the dependent variable in multiple regression, Arest was related to BMI and high screening BP, whereas Amst and ΔA were related to VO2peak and high screening BP ().
Table II. Plasma catecholamine and cardiovascular responses to mental stress.
Table III. Correlations between plasma adrenaline and peak oxygen uptake and measures of obesity in the combined groups.
Table IV. Stepwise multiple regression analyses with plasma adrenaline as the dependent variable.
Figure 1. Correlations between peak oxygen consumption and mean plasma adrenaline at rest (top left) and during mental stress test (MST) (top right), maximal level during MST (bottom left), and the response (bottom right). Filled circles and solid regression line: men with high (H) screening blood pressure; open circles and dashed regression line: men with normal (N) screening blood pressure. rs, Spearman's rank correlation coefficient. Correlations in H still significant after exclusion of the subject with the lowest peak oxygen consumption for mean (r = 0.59, p<0.05) and maximal (r = 0.66, p<0.01) adrenaline level during MST and for the response (rs = 0.57, p<0.05).
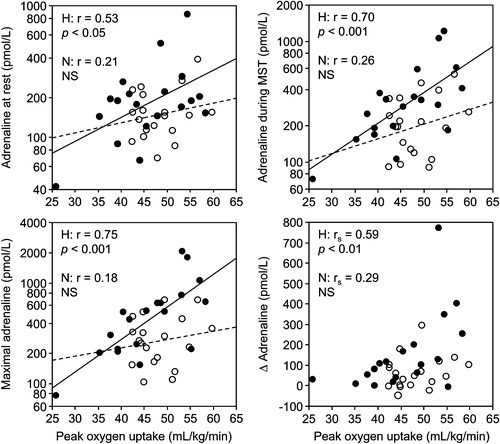
Figure 2. Correlations between body mass index and mean plasma adrenaline at rest (top) and during mental stress test (MST) (bottom). Filled circles and solid regression lines: men with high (H) screening blood pressure; open circles and dashed regression lines: men with normal (N) screening blood pressure.
Serum lipids and glucose metabolism in relation to fitness and adrenaline
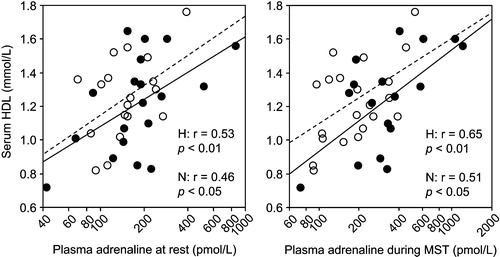
Serum triglyceride (rs = −0.37, p<0.05) and HDL (r = 0.44, p<0.01) levels correlated with VO2peak. In parallel, A levels correlated negatively with triglycerides and positively with HDL (, ). In multiple regression models adjusted for A levels, BMI was the only determinant of total cholesterol (β = 0.50, p<0.01) and triglyceride (β = 0.66, p<0.0001) levels. By contrast, HDL was predicted by waist circumference (β = −0.31, p<0.05) and Arest (β = 0.36, p<0.05), or, when added, by Amst alone (β = 0.55, p<0.001). Serum insulin correlated with VO2peak (rs = −0.43, p<0.01). It also correlated negatively with Amst (rs = −0.49, p<0.05) in the high screening BP group, but was independently related only to waist circumference (β = 0.78, p<0.0001) in this group, as in the overall regression analysis (β = 0.65, p<0.0001). Similarly, although GDR/I correlated with positively with Amst (r = 0.52, p<0.05) and ΔA (rs = 0.51, p<0.05), in men with high screening BP, it was predicted only by VO2peak in this group (β = 0.75, p<0.001), as in the overall analysis (β = 0.70, p<0.0001).
Table V. Correlations between plasma adrenaline and serum lipids and variables of glucose metabolism in the combined groups.
Figure 3. Correlations between mean plasma adrenaline level at rest (left) and during mental stress test (MST) (right) and serum high‐density lipoprotein cholesterol (HDL). Filled circles and solid regression line: men with high (H) screening blood pressure; open circles and dashed regression line: men with normal (N) screening blood pressure.
Sympathetic neural and cardiovascular responses in relation to fitness and adrenaline
NAmst and ΔNA were not significantly related to VO2peak. NAmst correlated with Amst (r = 0.54, p<0.001) and ΔNA with ΔA (rs = 0.71, p<0.0001), and these relationships remained significant (both β = 0.54, p<0.001) in multiple regression analysis. ΔHR correlated positively with VO2peak in the high screening BP group (r = 0.72, p<0.001). It correlated with ΔA in men with high (rs = 0.77, p<0.001) and normal screening BP (rs = 0.54, p<0.05). As the dependent variable in multiple regression, ΔHR was unrelated to VO2peak, whereas Amst (β = 0.70, p<0.0001), or, when added, ΔA (β = 0.76, p<0.0001) were the only independent predictors. ΔSBP correlated positively with VO2peak in men with high (r = 0.47, p<0.05) and normal (r = 0.61, p<0.01) screening BP. ΔDBP also correlated with VO2peak in the normal screening BP group (r = 0.59, p<0.01), but not significantly in the high screening BP group (r = 0.42, p = 0.08). As dependent variables in regression models, both ΔSBP (β = 0.54, p<0.001) and ΔDBP (β = 0.57, p<0.001) were independently related only to Amst.
Adrenaline levels during insulin and saline infusions versus baseline
In the 36 subjects with complete sets of blood samples during glucose clamp, plasma A fell from baseline to 30 min [232 (309) vs 158 (77) pmol/l, p<0.05] but then remained stable [181 (128) and 158 (87) pmol/l at 60 and 90 min, respectively]. The same occurred in the time control group, with plasma A levels 287 (90), 184 (90), 192 (92), and 186 (106) pmol/l at baseline, and 30, 60, and 90 min of saline infusion, respectively. Accordingly, there was no treatment×time interaction (p = 0.98).
Discussion
In these young men, high screening BP was associated with increased plasma A levels after adjustment for BMI and physical fitness. At the same time, resting A was negatively related to BMI, whereas the A level and response during mental stress were positively related to physical fitness, independently of BP status. Further, we found independent positive relationships between plasma A levels and serum HDL, but not with insulin sensitivity. The cardiovascular responses to mental stress were positively related to physical fitness, apparently as a result of higher A levels in more fit individuals.
To our knowledge, and as previously reviewed Citation[31], there has been little evidence that A responses to mental stress are related to physical fitness. Some studies Citation[31], Citation[38], Citation[39] have shown trends suggesting higher A during stress with higher fitness, but we are aware of only one report Citation[40] of a significant positive correlation. However, human and animal studies have suggested that the capacity to secrete A may increase as an adaptive response to physical training Citation[20]. For example, plasma A increases more in physically trained than in sedentary individuals at identical relative exercise intensities and in response to various non‐exercise stimuli Citation[20]. Also, athletes have been shown to have higher night‐time as well as 24‐h integrated plasma A levels than untrained subjects Citation[41]. Furthermore, the A content of the adrenal gland and the volume of the adrenal medulla are increased in endurance‐trained rats compared to sedentary control rats Citation[42]. Although our study did not involve physical training, the present observations suggest a similar effect on the response to psychological stress.
Circulating A mobilises substrates for energy metabolism by stimulating lipolysis, inhibiting insulin secretion, and increasing metabolic rate and thermogenesis Citation[43]. Several studies Citation[23], Citation[24], Citation[26–28] have suggested higher sympathetic neural activity, but lower adrenal medullary activity, with increasing measures of obesity. Also, subjects with more components of the metabolic syndrome were found to have progressively higher urinary NA excretion, but lower A excretion Citation[28]. The present observations support our previous findings Citation[3], Citation[10], Citation[25] of a negative association between BMI and A secretion at rest, but suggest that greater stress responses in lean subjects Citation[10] may at least partly be due to higher fitness. The correlations between A and lipid levels agree with findings from larger‐scale studies Citation[27], Citation[28] measuring 24‐h urinary A excretion. In particular, HDL was positively related to A excretion after adjustment for covariates, including BMI, serum insulin and physical activity Citation[27]. β‐adrenergic receptor blocking drugs decrease serum HDL and increase serum triglyceride levels, and these effects are lower for drugs with intrinsic sympathomimetic activity Citation[44]. Accordingly, low A secretion has been discussed as a possible contributor to the high triglyceride and low HDL levels associated with obesity and insulin resistance Citation[14], Citation[27], Citation[28]. However, we cannot exclude the possibility that the relationship reflects the known association between fitness and HDL Citation[17] through other mechanisms. There are previous reports Citation[25], Citation[27], Citation[28] in support of the negative correlation between plasma A and serum insulin in the men with high screening BP. On the other hand, the positive correlation between GDR/I and A in this group is not consistent with our previous findings Citation[8], Citation[45]. However, it was not significant when adjusted for VO2peak, suggesting poor fitness, and not adrenal medullary activity, as a main determinant of insulin resistance.
Studies of the relationship between fitness and plasma NA and cardiovascular responses to mental stress have shown inconsistent results Citation[30], Citation[31]. There are, however, some reports Citation[46–48] showing greater cardiovascular reactivity with higher fitness. In view of the present findings, it is tempting to suggest adrenal medullary activation as a link between fitness and cardiovascular reactivity. However, this interpretation relies on the assumption that endogenous A contributes significantly to such responses, which is not obvious Citation[1]. HR and BP reactivity are both dependent on cardiac β‐adrenergic responsiveness Citation[49], which was not assessed in the present study. Although A infusion increases plasma NA and HR Citation[50], Citation[51], the NA and HR responses to mental stress are not affected Citation[51], so the relationships between A levels and these variables do not necessarily imply direct causality. The BP response to mental stress is largely caused by an increase in cardiac output Citation[52]. Infusion of A increases stroke volume, ejection fraction and cardiac output Citation[50]. It induces a dose‐dependent Citation[50] and sustained Citation[53] increase in SBP, and increases the SBP response to mental stress Citation[51]. Thus, indirect evidence suggests a role for circulating A. However, positive correlations between VO2peak and BP responses might also be explained by increased adrenergic responsiveness with higher fitness. The inotropic response to β‐adrenergic stimulation is enhanced in endurance athletes Citation[54] and increased by exercise training Citation[55]. Accordingly, higher fitness has been associated with greater SBP responses to A Citation[56].
Some limitations of the present study should be mentioned. First, the cross‐sectional design does not allow for definite mechanistic conclusions. The data should also be viewed with some caution due to the relatively small sample size, and the large number of statistical tests increasing the risk of chance findings. We did not recruit men with screening BP between 115/75 and 140/90 mmHg, who might be more representative of young men in general. However, correlations which were rather similar in our two groups (e.g. between A and HDL and BMI) might not differ radically in men with intermediate BP levels. Limitations to the use of venous plasma catecholamine concentrations include disproportionate reflection of local sympathetic nerve activity by NA and lower A concentrations than in arterial blood (i.e. those to which tissues are exposed) Citation[1]. Finally, as discussed previously Citation[11], blood sampling during hyperinsulinaemia is a potential limitation of the present work. However, in our studies Citation[9], Citation[57], the glucose clamp has not affected resting plasma NA or HR more than saline infusion, nor has it affected cardiovascular or catecholamine responses to MST Citation[9]. In agreement with our previous observations Citation[9], Citation[57], plasma A was not affected by insulin compared to saline infusion. Rather, the reduction during both infusions suggests a similar adaptation to supine rest. Thus, the correlations with metabolic variables were similar for plasma A at baseline and the average resting level.
As discussed previously Citation[7], high screening BP during military enlistment may partly reflect increased BP reactivity to stress. The present findings add to our previous studies Citation[7–11], Citation[45] by suggesting that cardiovascular reactivity in young men can be affected by physical fitness through differences in adrenal medullary activity. It may seem like a paradox that increased A secretion has potentially harmful Citation[2] and pro‐hypertensive Citation[4] cardiovascular effects, but is associated with metabolic characteristics known to protect against hypertension and/or its consequences. However, not all hypertensive patients have metabolic abnormalities Citation[14]. The assumed pro‐hypertensive effect of high A secretion may be difficult to prove if it is counterbalanced by a beneficial metabolic profile and efficient vagal cardiac control.
In conclusion, our findings suggest a positive relationship between physical fitness and adrenal medullary activity during mental stress in young men. This may contribute to the proposed relationship between low A secretion and low HDL cholesterol in obesity Citation[14], which is also supported by our findings. However, plasma A was independently associated with high screening BP and closely related to plasma NA and cardiovascular stress responses. Increased adrenaline secretion may be related to high BP, but may at the same time be associated with high physical fitness and a beneficial metabolic profile.
Acknowledgements
We thank the Norwegian Council on Cardiovascular Diseases for financial support, and the Joint Norwegian Medical Services, Head Quarters of the Defence Command, Norway, for their generous cooperation.
References
- Hjemdahl P. Plasma catecholamines – analytical challenges and physiological limitations. Baillieres Clin Endocrinol Metab 1993; 7: 307–353
- Wortsman J. Role of epinephrine in acute stress. Endocrinol Metab Clin North Am 2002; 31: 79–106
- Kjeldsen S. E., Schork N. J., Leren P., Eide I. K. Arterial plasma norepinephrine correlates to blood pressure in middle‐aged men with sustained essential hypertension. Am Heart J 1989; 118: 775–781
- Floras J. S. Epinephrine and the genesis of hypertension. Hypertension 1992; 19: 1–18
- Jacobs M. C., Lenders J. W., Willemsen J. J., Thien T. Adrenomedullary secretion of epinephrine is increased in mild essential hypertension. Hypertension 1997; 29: 1303–1308
- Rumantir M. S., Jennings G. L., Lambert G. W., Kaye D. M., Seals D. R., Esler M. D. The ‘adrenaline hypothesis’ of hypertension revisited: Evidence for adrenaline release from the heart of patients with essential hypertension. J Hypertens 2000; 18: 717–723
- Rostrup M., Westheim A., Kjeldsen S. E., Eide I. Cardiovascular reactivity, coronary risk factors, and sympathetic activity in young men. Hypertension 1993; 22: 891–899
- Moan A., Nordby G., Rostrup M., Eide I., Kjeldsen S. E. Insulin sensitivity, sympathetic activity and cardiovascular reactivity in young men. Am J Hypertens 1995; 8: 268–275
- Høieggen A., Fossum E., Moan A., Rostrup M., Eide I. K., Kjeldsen S. E. Effects of hyperinsulinemia on sympathetic responses to mental stress. Am J Hypertens 2000; 13: 21–28
- Reims H. M., Fossum E., Høieggen A., Moan A., Eide I., Kjeldsen S. E. Adrenal medullary overactivity in lean borderline hypertensive young men. Am J Hypertens 2004; 17: 611–618
- Reims H. M., Sevre K., Fossum E., Høieggen A., Eide I., Kjeldsen S. E. Plasma catecholamines, blood pressure responses and perceived stress during mental arithmetic stress in young men. Blood Press 2004; 13: 287–294
- Brown M. J., Macquin I. Is adrenaline the cause of essential hypertension?. Lancet 1981; 318: 1079–1082
- Treiber F. A., Kamarck T., Schneiderman N., Sheffield D., Kapuku G., Taylor T. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosom Med 2003; 65: 46–62
- Reaven G. M., Lithell H., Landsberg L. Hypertension and associated metabolic abnormalities – the role of insulin resistance and the sympathoadrenal system. N Engl J Med 1996; 334: 374–381
- Hall J. E., Hildebrandt D. A., Kuo J. Obesity hypertension: Role of leptin and sympathetic nervous system. Am J Hypertens 2001; 14((6 Pt 2))103S–115S
- Hu G., Barengo N. C., Tuomilehto J., Lakka T. A., Nissinen A., Jousilahti P. Relationship of physical activity and body mass index to the risk of hypertension: A prospective study in Finland. Hypertension 2004; 43: 25–30
- Carnethon M. R., Gidding S. S., Nehgme R., Sidney S., Jacobs D. R Jr., Liu K. Cardiorespiratory fitness in young adulthood and the development of cardiovascular disease risk factors. JAMA 2003; 290: 3092–3100
- Alvarez G. E., Halliwill J. R., Ballard T. P., Beske S. D., Davy K. P. Sympathetic neural regulation in endurance‐trained humans: Fitness vs. fatness. J Appl Physiol 2005; 98: 498–502
- Buch A. N., Coote J. H., Townend J. N. Mortality, cardiac vagal control and physical training – what's the link?. Exp Physiol 2002; 87: 423–435
- Kjær M. Adrenal medulla and exercise training. Eur J Appl Physiol 1998; 77: 195–199
- Del Rio G. Adrenomedullary function and its regulation in obesity. Int J Obes Relat Metab Disord 2000; 24(Suppl 2)S89–S91
- Peterson H. R., Rothschild M., Weinberg C. R., Fell R. D., McLeish K. R., Pfeifer M. A. Body fat and the activity of the autonomic nervous system. N Engl J Med 1988; 318: 1077–1083
- Leonetti D. L., Bergstrom R. W., Shuman W. P., Wahl P. W., Jenner D. A., Harrison G. A. Urinary catecholamines, plasma insulin and environmental factors in relation to body fat distribution. Int J Obes 1991; 15: 345–357
- Troisi R. J., Weiss S. T., Parker D. R., Sparrow D., Young J. B., Landsberg L. Relation of obesity and diet to sympathetic nervous system activity. Hypertension 1991; 17: 669–677
- Os I., Kjeldsen S. E., Nordby G., Eide I., Lande K., Hjermann I. Sex differences in essential hypertension. J Intern Med 1993; 233: 13–19
- Scherrer U., Randin D., Tappy L., Vollenweider P., Jequier E., Nicod P. Body fat and sympathetic nerve activity in healthy subjects. Circulation 1994; 89: 2634–2640
- Ward K. D., Sparrow D., Landsberg L., Young J. B., Vokonas P. S., Weiss S. T. The relationship of epinephrine excretion to serum lipid levels: The Normative Aging Study. Metabolism 1994; 43: 509–513
- Lee Z. S., Critchley J. A., Tomlinson B., Young R. P., Thomas G. N., Cockram C. S. Urinary epinephrine and norepinephrine interrelations with obesity, insulin, and the metabolic syndrome in Hong Kong Chinese. Metabolism 2001; 50: 135–143
- Crews D. J., Landers D. M. A meta‐analytic review of aerobic fitness and reactivity to psychosocial stressors. Med Sci Sports Exerc 1987; 19(Suppl 5)S114–S120
- Claytor R. P. Stress reactivity: Hemodynamic adjustments in trained and untrained humans. Med Sci Sports Exerc 1991; 23: 873–881
- Sothmann M. S., Hart B. A., Horn T. S. Plasma catecholamine response to acute psychological stress in humans: Relation to aerobic fitness and exercise training. Med Sci Sports Exerc 1991; 23: 860–867
- Hill J. O., Melanson E. L. Overview of the determinants of overweight and obesity: Current evidence and research issues. Med Sci Sports Exerc 1999; 31(Suppl 1)S515–S521
- Reims H. M., Sevre K., Fossum E., Høieggen A., Mellem H., Kjeldsen S. E. Relations between insulin sensitivity, fitness, and autonomic cardiac regulation in healthy, young men. J Hypertens 2004; 22: 2007–2015
- Imholz B. P., Wieling W., van Montfrans G. A., Wesseling K. H. Fifteen years experience with finger arterial pressure monitoring: Assessment of the technology. Cardiovasc Res 1998; 38: 605–616
- Tanaka H., Monahan K. D., Seals D. R. Age‐predicted maximal heart rate revisited. J Am Coll Cardiol 2001; 37: 153–156
- Peuler J. D., Johnson G. A. Simultaneous single isotope radioenzymatic assay of plasma norepinephrine, epinephrine and dopamine. Life Sci 1977; 21: 625–636
- Kjeldsen S. E., Flaaten B., Eide I., Helgeland A., Leren P. Evidence of increased peripheral catecholamine release in patients with long‐standing, untreated essential hypertension. Scand J Clin Lab Invest 1982; 42: 217–223
- Sinyor D., Schwartz S. G., Peronnet F., Brisson G., Seraganian P. Aerobic fitness level and reactivity to psychosocial stress: Physiological, biochemical, and subjective measures. Psychosom Med 1983; 45: 205–217
- Claytor R. P., Cox R. H., Howley E. T., Lawler K. A., Lawler J. E. Aerobic power and cardiovascular response to stress. J Appl Physiol 1988; 65: 1416–1423
- Sothmann M. S., Gustafson A. B., Garthwaite T. L., Horn T. S., Hart B. A. Cardiovascular fitness and selected adrenal hormone responses to cognitive stress. Endocr Res 1988; 14: 59–69
- Dela F., Mikines K. J., Von Linstow M., Galbo H. Heart rate and plasma catecholamines during 24 h of everyday life in trained and untrained men. J Appl Physiol 1992; 73: 2389–2395
- Stallknecht B., Kjær M., Mikines K. J., Maroun L., Ploug T., Ohkuwa T. Diminished epinephrine response to hypoglycemia despite enlarged adrenal medulla in trained rats. Am J Physiol 1990; 259: R998–R1003
- Webber J., Macdonald I. A. Metabolic actions of catecholamines in man. Baillière's Clin Endocrinol Metab 1993; 7: 393–413
- Kasiske B. L., Ma J. Z., Kalil R. S., Louis T. A. Effects of antihypertensive therapy on serum lipids. Ann Intern Med 1995; 122: 133–141
- Reims H., Høieggen A., Fossum E., Eide I., Kjeldsen S. E. Glucose disposal rates calculated from 60‐90 min isoglycaemic hyperinsulinaemic glucose clamp correlate with cardiovascular risk factors in borderline hypertensive, but otherwise healthy, young men. Metabolism 2001; 50: 1175–1180
- de Geus E. J., van Doornen L. J., Orlebeke J. F. Regular exercise and aerobic fitness in relation to psychological make‐up and physiological stress reactivity. Psychosom Med 1993; 55: 347–363
- Dishman R. K., Jackson E. M., Nakamura Y. Influence of fitness and gender on blood pressure responses during active or passive stress. Psychophysiology 2002; 39: 568–576
- Dishman R. K., Nakamura Y., Jackson E. M., Ray C. A. Blood pressure and muscle sympathetic nerve activity during cold pressor stress: Fitness and gender. Psychophysiology 2003; 40: 370–380
- Eisenhofer G., Lambie D. G., Johnson R. H. Beta‐adrenoceptor responsiveness and plasma catecholamines as determinants of cardiovascular reactivity to mental stress. Clin Sci 1985; 69: 483–492
- Stratton J. R., Pfeifer M. A., Ritchie J. L., Halter J. B. Hemodynamic effects of epinephrine: Concentration‐effect study in humans. J Appl Physiol 1985; 58: 1199–1206
- Jern S., Pilhall M., Jern C. Infusion of epinephrine augments pressor responses to mental stress. Hypertension 1991; 18: 467–474
- Brod J., Fencl V., Hejl Z., Jirka J. Circulatory changes underlying blood pressure elevation during acute emotional stress (mental arithmetic) in normotensive and hypertensive subjects. Clin Sci 1959; 18: 269–279
- Blankestijn P. J., Man in't Veld A. J., Tulen J., van den Meiracker A. H., Boomsma F., Moleman P. Support for adrenaline‐hypertension hypothesis: 18 hour pressor effect after 6 hours adrenaline infusion. Lancet 1988; 332: 1386–1389
- Hopkins M. G., Spina R. J., Ehsani A. A. Enhanced beta‐adrenergic‐mediated cardiovascular responses in endurance athletes. J Appl Physiol 1996; 80: 516–521
- Mier C. M., Turner M. J., Ehsani A. A., Spina R. J. Cardiovascular adaptations to 10 days of cycle exercise. J Appl Physiol 1997; 83: 1900–1906
- Svedenhag J., Martinsson A., Ekblom B., Hjemdahl P. Altered cardiovascular responsiveness to adrenaline in endurance‐trained subjects. Acta Physiol Scand 1986; 126: 539–550
- Moan A., Høieggen A., Nordby G., Birkeland K., Eide I., Kjeldsen S. E. The glucose clamp procedure activates the sympathetic nervous system even in the absence of hyperinsulinemia. J Clin Endocrinol Metab 1995; 80: 3151–3154