Abstract
Background. The aim of this study was to investigate the impact of short‐term treatment with the angiotensin II receptor blocker (ARB) valsartan on retinal endothelial function in elderly patients with mild to moderate essential hypertension. Methods. In an open‐labeled study, 20 elderly, male patients with arterial hypertension (WHO I–II) were treated with the ARB valsartan (80–160 mg once daily) over 8 days. Central retinal artery perfusion at rest and during flicker light stimulation was measured before and after treatment using pulsed wave Doppler sonography. Retinal capillary flow was assessed with scanning laser Doppler flowmetry at rest and following systemic infusion of the nitric oxide synthase (NOS) inhibitor NG‐monomethyl‐l‐arginine (L‐NMMA). Results. While valsartan significantly lowered blood pressure, central retinal artery perfusion at rest as well as after flicker light stimulation was similar before and after treatment. Similarly, retinal capillary flow at rest and after infusion of L‐NMMA did not change with valsartan after 7 days of treatment. Subgroup analysis revealed that changes in retinal capillary flow in response to L‐NMMA might be dependent on serum low‐density lipoprotein (LDL) cholesterol levels of study participants. After treatment with valsartan, retinal capillary flow in response to L‐NMMA decreased more in patients with low (<3.54 mmol/l) than with high LDL‐cholesterol levels (−12.6±20.2% vs 12.3±19.5%, p<0.05). Conclusion. Short‐term treatment with valsartan did not improve retinal endothelial function in elderly hypertensive patients.
Introduction
Hypertension is a major risk factor for ischemic stroke Citation[1]. Treatment with angiotensin II receptor blockers (ARBs) can prevent stroke in hypertensive patients and there is accumulating evidence indicating that cardiovascular risk reduction with ARBs goes beyond the blood pressure (BP) lowering properties of these agents Citation[2,3].
Experimental stroke models in animals have demonstrated that pretreatment with ARBs reduces neuronal injury in response to cerebral artery occlusion by improving arterial compliance and cerebral blood flow during ischemia in areas distal to the site of occlusion Citation[4]. Clinical studies in humans have shown that ARBs reduce intima‐media thickness and restore compliance of arteries Citation[5,6]. However, the impact of ARBs on cerebral perfusion in humans remains largely unknown.
Given the difficulties of direct assessment of cerebral blood flow in humans, surrogate markers of cerebral perfusion such as retinal perfusion have become the focus of recent research. The retinal vasculature is morphologically related to cerebral blood vessels due to the common origin from the internal carotid artery and several studies have shown close functional similarities between both vasculatures Citation[7]. Since direct visualization of retinal blood vessels is readily achievable, non‐invasive studies of the retinal vasculature may serve as a novel tool to analyze cerebral vascular function in humans.
In this context we and others have recently validated a simple reproducible method to directly measure retinal perfusion in humans by use of laser Doppler Citation[8–10].
Based upon our previous observation that short‐term treatment with the ARB candesartan improved retinal endothelial function in young hypertensive males Citation[8], the aim of the present pilot study was to test the effect of an ARB on retinal endothelial function in elderly hypertensive individuals.
Methods
Study cohort
Study participants were recruited by advertisement in a local newspaper. Subjects eligible for the study were referred to our outpatient clinic. Inclusion criteria were age (50–80 years), gender (male), ethnicity (Caucasian) and mild to moderate essential hypertension (WHO I–II). Patients with diabetes, nicotine abuse and those being treated with either an angiotensin‐converting enzyme inhibitor or an ARB for BP control, as well as subjects with any eye disease other than grade I hypertensive retinopathy or clinically relevant differences in vision between both eyes, were excluded. Secondary forms of hypertension were ruled out by routine clinical examination, ultrasound studies including sonography of the kidneys and adrenal glands as well as duplex sonography of renal arteries and a routine panel of laboratory tests.
All patients were offered outpatient care by our department. In addition, participants were examined at the outpatient clinic of the Department of Ophthalmology to evaluate the ocular exclusion criteria.
BP was measured after 5 min of rest in sitting position; the cuff size was adjusted according to the arm circumference. Three consecutive BP readings obtained at two different occasions at least 2 weeks apart were performed and average BP was calculated. Patients were enrolled into the study if the screening office BP exceeded the threshold level of either 140 mmHg systolic or 90 mmHg diastolic (following the guidelines of the European Society of Hypertension 2003). Sitting BP on average was 163±16/97±7 mmHg. A total of 20 patients met the study inclusion criteria and participated in the study.
Written informed consent was obtained prior to study inclusion and the study was approved by the local Ethical Committee of the University of Erlangen‐Nürnberg.
Study protocol
Baseline measurements of central retinal artery and retinal capillary perfusion were performed as described below. Patients were then instructed to take the ARB valsartan once daily over a period of 8 days (80 mg for 4 days followed by 160 mg for 4 days). Following pharmacological intervention, intraocular measurements were repeated. All measurements were performed in a quiet, temperature‐ and light‐controlled room during afternoon hours (i.e. between 14.00 and 16.00 h). For each time point, the following tests were performed.
Measurement of central retinal artery blood flow velocity
Blood flow velocity within the right central retinal artery was measured using pulsed Doppler sonography at 4 MHz (EME Companion; Nicolet Biomedical Inc., Madison, WI, USA) as described earlier Citation[8,9]. The spatial peak temporal average intensity of the transducer was 821 mW/cm2. The mean sampling depth was 30±4 mm, with a gate (measuring distance) of 5 mm. The running pulse curve and electrocardiogram (Mennen Medical 740; Schubart, Wiesbaden, Germany) were simultaneously recorded over 3 min and stored on a computer. At least 10 pulse curves were averaged for quantitative analysis using an automated computer analysis software program (Flicker version 1.0; J. T. Harazny, Erlangen, Germany). Analysis comprised systolic maximum, end‐diastolic minimum and mean blood flow velocities.
All measurements were performed with study participants in supine position after 30 min of rest. Blood flow velocities in the central retinal artery were measured at baseline, during flicker light stimulation (recordings started 5–20 s after the start of luminance flicker) and 10–15 min after flicker light exposure. For flicker light stimulation (flicker frequency 10 Hz) during pulsed Doppler sonography examinations, a commercially available device (Photo Stimulator 750; Siemens‐Elema AB, Solna, Sweden) was used. The lamp was mounted 20 cm away from the right eye and patients were instructed to focus on the lamp while measurements were performed. Flicker light stimulation has been shown to increase retinal blood flow in part via a nitric oxide (NO)‐dependent mechanism Citation[11–14]. Accordingly, flicker light induced changes in blood flow velocities were used as a test to study NO‐dependent retinal endothelial function and its impact on retinal blood flow.
Measurement of retinal capillary flow
Upon completion of assessment of retinal blood flow velocities, patients were placed in the sitting position and baseline retinal capillary flow was measured after a 30‐min resting period. Retinal capillary blood flow was measured using scanning laser Doppler flowmetry at 670 nm (Heidelberg Retina Flowmeter; Heidelberg Engineering, Heidelberg, Germany), as previously described in detail Citation[8].
Briefly, the Doppler shift in a retinal sample of 2.56×0.64×0.30 mm was scanned within 2 s at a resolution of 256 points×64×128 lines. The confocal technique of the device ensured that only the capillary flow of the superficial retinal layer of 300 µm was measured. Measurements were performed in the juxtapapillary area of the right eye, 2–3 mm temporally to the optic nerve. All measurements with scanning laser Doppler flowmetry were performed in triplicate and mean values were computed. Analysis of perfusion images was performed offline with automatic full field analysis (SLDF version 3.3; Heidelberg Engineering, Heidelberg, Germany) Citation[8]. This led to a perfusion map excluding vessels with a diameter greater than 30 µm, with lines with saccades, and with pixels of inadequate reflectivity. Mean retinal capillary flow was calculated in the area of interest and expressed as arbitrary units (AU).
Following baseline measurements, the NO synthase inhibitor NG‐monomethyl‐l‐arginine (L‐NMMA) was administered intravenously as a bolus infusion over 5 min (3 mg/kg, Clinalfa, Basel, Switzerland). Immediately after L‐NMMA infusion, retinal capillary flow measurements were repeated. For safety reasons, all participants received an infusion of 100 mg/kg l‐arginine (University Pharmacy, Erlangen, Germany) immediately after measurements were performed to overcome NO synthase inhibition by excess substrate supply for NO synthesis. The applied dose of 100 mg/kg has been previously shown to reverse the effects of L‐NMMA in other vascular beds Citation[15].
Prior and during all provocative maneuvers, mean arterial blood pressure (MAP) was recorded every 5 min with an oscillometric device (Dinamap, Critikon, Norderstedt, Germany).
Statistical analysis
All statistical analysis was carried out using SPSS software (release 10.0; SPSS Inc., Chicago, IL). Paired and unpaired Student's t‐test and covariance analysis were applied for comparisons where appropriate. A two‐tailed p‐value <0.05 was considered significant. All values are expressed as mean±standard deviation.
Results
Study population
A total of 20 male subjects were enrolled in this study. Mean age of study participants was 60±7 years, pretreatment systolic BP was 144±15 mmHg, mean diastolic BP 89±10 mmHg. Of the 20 hypertensive patients, 15 received antihypertensive medication consisting of calcium antagonists (n = 7), beta‐blockers (n = 11) or diuretics (n = 3). Of note, concomitant medication was not changed throughout the study period.
Study participants were slightly obese (body mass index 27.6±4.1 kg/m2), fasting low‐ (LDL) and high‐density lipoprotein (HDL) cholesterol levels were 3.54±0.9 mmol/l and 1.35±0.3 mmol/l, respectively, and fasting triglyceride levels were 2.1±1.5 mmol/l.
Valsartan and central retinal artery blood flow velocity
Despite significant reductions in MAP (107±11 vs 103±11 mmHg, p<0.05) valsartan did not change peak systolic, peak diastolic and mean blood flow velocities in the central retinal artery (). Similarly, valsartan did not alter retinal capillary flow ().
Table I. Effect of valsartan on systemic and retinal hemodynamics.
Table II. Effect of valsartan on retinal perfusion following flicker light exposure and NG‐monomethyl‐l‐arginine (L‐NMMA) infusion.
Effect of flicker light and L‐NMMA on retinal and systemic hemodynamics
The impact of flicker light exposure on MAP or central retinal artery blood flow velocities was not altered by valsartan treatment. Flicker light increased mean blood flow velocity by 29±26% (before) vs 37±41% (after valsartan, p = n.s.), whereas MAP increased by 2.3±7.3 mmHg (before) vs 1.0±4.7 mmHg (after valsartan, p = n.s., ). L‐NMMA infusion significantly increased MAP by 12±6 mmHg (p<0.001) and this rise was similar before and after treatment with valsartan ().
As with flicker light exposure, central retinal artery blood flow velocities as well as retinal capillary blood flow did not change after L‐NMMA infusion and were not affected by valsartan treatment ().
Impact of LDL‐cholesterol on retinal and systemic hemodynamics
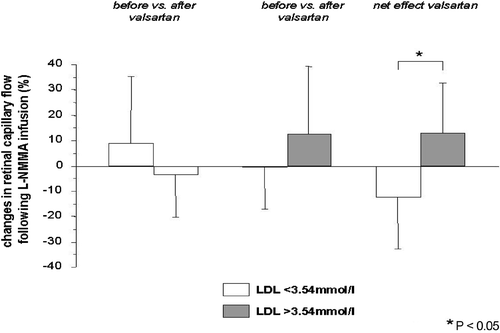
To identify determinants of the response in retinal capillary flow to L‐NMMA infusion, we conducted a multivariate analysis, with specific attention to the role of cardiovascular risk factors other than high BP. LDL‐cholesterol concentrations seemed to be of some importance (p = 0.095), whereas age (p = 0.36) and systolic BP (p = 0.18) did not. Although there was no significant result in this analysis, LDL‐cholesterol level seems to be important to predict the reaction of the retinal capillary bed after L‐NMMA. Subsequently, we divided the study population into two groups based on the median of LDL‐cholesterol levels (LDL<3.54 mmol/l vs LDL>3.54 mmol/l, each n = 10) and the response to flicker light exposure and L‐NMMA infusion was reanalyzed.
While baseline retinal hemodynamics were similar between patients with low (<3.54 mmol/l) vs high (>3.54 mmol/l) LDL‐cholesterol (), the valsartan induced changes in retinal capillary flow in response to L‐NMMA infusion were significantly different between subjects with high vs low LDL‐cholesterol (p = 0.012). Following treatment with valsartan, retinal capillary flow in response to L‐NMMA changed by 12.3±19.5% in patients with high (>3.54 mmol/l) LDL‐cholesterol, whereas it decreased by −12.6±20.2% in patients with low (<3.54 mmol/l) LDL‐cholesterol (p = 0.012, , ).
Table III. Baseline retinal perfusion before treatment with valsartan stratified upon low‐density lipoprotein (LDL)‐cholesterol levels.
Table IV. Impact of valsartan on retinal perfusion in response to flicker light exposure and NG‐monomethyl‐l‐arginine (L‐NMMA) infusion in patients with low and high low‐density lipoprotein (LDL)‐cholesterol.
Discussion
In this study, short‐term treatment with the ARB valsartan in elderly hypertensive patients failed to improve retinal endothelial function measured as changes in central retinal artery blood flow velocity and retinal capillary blood flow. In particular, neither the response to flicker light exposure nor that to systemic infusion of L‐NMMA was influenced by valsartan treatment despite a significant reduction in BP.
An increase in NO bioavailability and restoration of impaired endothelium‐dependent vasodilation through ARBs has been described in various vascular beds including the forearm, coronary and renal circulation Citation[16–19]. Coherently with these observations, we have recently shown that the ARB candesartan improves retinal endothelial function in young hypertensive males Citation[8]. In these studies, young (18–35 years), normotensive and age‐matched hypertensive males underwent testing of retinal endothelial function. In young normotensive males, L‐NMMA infusion elicited a significant decrease in retinal capillary flow of ∼10%, which remained unaltered after short‐term treatment with the ARB candesartan (16 mg once daily over 8 days). In contrast, and similarly to the observation of the present study, L‐NMMA infusion did not change retinal capillary flow in young hypertensive males prior to treatment with candesartan, whereas 1 week of treatment led to a significant decrease in retinal capillary flow following L‐NMMA infusion, indicating that candesartan treatment improved retinal endothelial function. Interestingly, despite a similar treatment duration neither the response to flicker light stimulation nor that to L‐NMMA infusion was influenced by the ARB valsartan in the present study in which elderly hypertensive patients were analyzed.
Despite the lack of a response to valsartan treatment regarding the entire study population, subgroup analysis revealed a significant effect of valsartan on retinal capillary flow in response to L‐NMMA infusion if patients were stratified according to their baseline LDL‐cholesterol levels. Thus, in patients with high LDL‐cholesterol levels (>3.54 mmol/l) retinal capillary flow in response to L‐NMMA increased by as much as 12.3% after treatment with valsartan, whereas it decreased by −12.6% in patients with low LDL‐cholesterol levels (<3.54 mmol/l). By contrast, central retinal blood flow velocities in response to flicker light exposure or L‐NMMA infusion were not dependent upon LDL‐cholesterol levels.
To summarize, the findings of the subgroup analysis indicate an impaired endothelial function of the retinal capillary vasculature in hypertensive individuals with low LDL‐cholesterol levels that could be improved after short‐term treatment with valsartan. However, this is a post‐hoc analysis with rather low number of subjects. Clearly, we consider this finding a hypothesis generating results that need subsequent evaluations.
Our study is limited in that it was neither randomized nor placebo‐controlled and did not include a normotensive control group. However, this was not the aim of the present study, which had the sole intention of investigating the impact of short‐term treatment with an ARB on retinal endothelial function in a well‐characterized study cohort with established essential hypertension. Our results are limited in that we did not analyze the impact of valsartan on retinal parameters at a later time point. Furthermore, given the short, yet intended, treatment period we could not titrate the study medication to the maximum dose of 320 mg/day. Thus, although valsartan significantly lowered BP after 8 days of treatment, we do not know whether duration or treatment doses were sufficient to achieve relevant drug concentrations within the cerebral circulation. While we have previously shown that short‐term treatment with the ARB candesartan in young hypertensive males significantly improves retinal endothelial function, one may speculate about a different potency of these two ARBs. However, given the well‐characterized pharmacological profiles of ARBs, which so far indicate equal potency, i.e. non‐superiority of a certain drug member, we think it is more likely that age‐related differences may account for the observed discrepancies of ARBs on retinal endothelial function in young vs elderly hypertensive patients. Previously, age‐dependent effects on endothelial function have been reported in the peripheral as well as renal circulation [20,21] and we suggest that this age‐dependency of NO activity extends also to the retinal circulation.
In conclusion, in the present study we failed to observe an effect of short‐term treatment with the ARB valsartan on parameters of retinal endothelial function in male, elderly hypertensive patients. However, stratification of patients according to another cardiovascular risk factor, namely high LDL‐cholesterol, revealed that with the double burden of hypertension and high cholesterol levels (>3.54 mmol/l) NO activity did not change after 7 days of treatment by the angiotensin receptor blocker valsartan as in contrast to patients with low LDL‐cholesterol levels (<3.54 mmol/l).
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft (KFG 106‐1 TP4) and Novartis Pharma GmbH. The authors are indebted to Johannes Jacobi for critical comments and revision of the manuscript.
References
- Bronner L. L., Kanter D. S., Manson J. E. Primary prevention of stroke. N Engl J Med 1995; 333: 1392–1400
- Julius S., Kjeldsen S. E., Weber M., Brunner H. R., Ekman S., Hansson L., , VALUE trial group, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: The VALUE randomised trial. Lancet 2004; 363: 2022–2031
- Dahlof B., Devereux R. B., Kjeldsen S. E., Julius S., Beevers G., de Faire U., , LIFE Study Group, et al. Cardiovascular morbidity and mortality in the Losartan Intervention FOR Endpoint reduction in hypertension study (LIFE): A randomised trial against atenolol. Lancet 2002; 359: 995–1003
- Ito T., Yamakawa H., Bregonzio C., Terron J. A., Falcon‐Neri A., Saavedra J. M. Protection against ischemia and improvement of cerebral blood flow in genetically hypertensive rats by chronic pretreatment with an AT1 antagonist. Stroke 2002; 33: 2297–2303
- Ludwig M., Stapff M., Ribeiro A., Fritschka E., Tholl U., Smith R. D., et al. Comparison of the effect of losartan and atenolol on common carotid artery intima‐media thickness in patients with hypertension. Clin Ther 2002; 24: 1175–1193
- Uchiyama‐Tanaka Y., Mori Y., Kishimoto N., Fukui M., Nose A., Kijima Y., et al. Comparison of the effects of quinapril and losartan on carotid artery intima‐media thickness in patients with mild‐to‐moderate arterial hypertension. Kidney Blood Press Res 2005; 28: 111–116
- Hilton E. J., Hosking Sl., Betts T. Epilepsy patients treated with antiepileptic drug exhibit compromised ocular perfusion characteristics. Epilepsia 2002; 43: 1346–1350
- Delles C., Michelson G., Harazny J., Oehmer S., Hilgers K. F., Schmieder R. E. Impaired endothelial function of the retinal vasculature in hypertensive patients. Stroke 2004; 35: 1289–1293
- Michelson G., Welzenbach J., Pal I., Harazny J. Automatic full field analysis of perfusion images gained by scanning laser Doppler flowmetry. Br J Ophthalmol 1998; 82: 1294–1300
- Michelson G., Patzelt A., Harazny J. Flickering light increases retinal blood flow. Retina 2002; 22: 336–343
- Kondo M., Wang L., Bill A. The role of nitric oxide in hyperaemic response to flicker in the retina and optic nerve in cats. Acta Ophthalmol Scand 1997; 75: 232–235
- Donati G., Pournaras C. J., Munoz J. L., Poitry S., Poitry‐Yamate C. L., Tsacopoulos M. Nitric oxide controls arteriolar tone in the retina of the miniature pig. Invest Ophthalmol Vis Sci 1995; 36: 2228–2237
- Buerk D. G., Riva C. E., Cranstoun S. D. Nitric oxide has a vasodilatory role in cat optic nerve head during flicker stimuli. Microvasc Res 1996; 52: 13–26
- Dorner G. T., Garhofer G., Kiss B., Polska E., Polak K., Riva C. E., et al. Nitric oxide regulates retinal vascular tone in humans. Am J Physiol Heart Circ Physiol 2003; 285: H631–H636
- Mayer B. X., Mensik C., Krishnaswami S., Derendorf H., Eichler H. G., Schmetterer L., et al. Pharmacokinetic–pharmacodynamic profile of systemic nitric oxide‐synthase inhibition with L‐NMMA in humans. Br J Clin Pharmacol 1999; 47: 539–544
- Delles C., Jacobi J., Schlaich M. P., John S., Schmieder R. E. Assessment of endothelial function of the renal vasculature in human subjects. Am J Hypertens 2002; 15: 3–9
- Delles C., Jacobi J., John S., Fleischmann I., Schmieder R. E. Effects of enalapril and eprosartan on the renal vascular nitric oxide system in human essential hypertension. Kidney Int 2002; 61: 1462–1468
- Ghiadoni L., Virdis A., Magagna A., Taddei S., Salvetti A. Effect of the angiotensin II type 1 receptor blocker candesartan on endothelial function in patients with essential hypertension. Hypertension 2000; 35: 501–506
- Prasad A., Halcox J. P., Waclawiw M. A., Quyyumi A. A. Angiotensin type 1 receptor antagonism reverses abnormal coronary vasomotion in atherosclerosis. J Am Coll Cardiol 2001; 38: 1089–1095