Abstract
The relationship between blood pressure (BP) variability and stroke location was examined in 85 patients admitted with acute ischemic stroke. The patients were divided into three groups according to stroke location: right hemisphere (32 patients), left hemisphere (30 patients) and non‐localized (23 patients). BP upon admission was 147.94/76.53±20.72/13.70 mmHg in the right hemisphere group, 151.81/76.10±25.69/16.23 mmHg in the left hemisphere and 155.23/83.41±30.45/15.74 in the non‐localized group. The left hemisphere group had significantly (p<0.01) greater variations in systolic and diastolic BP between days 2 and 3 and in systolic BP between days 3 and 4 after stroke compared with the other groups. BP in the left hemisphere group was less stable than in the other two groups. Non‐localized patients without pre‐existing hypertension had a significantly lower and more stable BP during the week following stroke than non‐localized patients with pre‐existing hypertension. Non‐localized patients with pre‐existing hypertension had the highest BP and showed no improvement during the week. Systolic BP tended to be higher and less stable in left hemisphere patients than in right hemisphere, whereas among non‐localized ischemic stroke patients BP was higher in those who had a prior diagnosis of hypertension.
Introduction
Stroke is the third most common cause of death following coronary heart disease and cancer, and occurs with greater frequency in patients with myocardial infarction and hypertension. It is the most common life‐threatening neurological disorder and the most important single cause of disability in western populations Citation[1]. Each year, 30.9 million individuals worldwide experience stroke, being responsible for approximately 4 million deaths. High blood pressure (BP) is a serious risk factor for stroke Citation[1]. Despite intensive research efforts, there are few effective treatments once stroke has occurred Citation[2].
Hypertension is a common finding in patients hospitalized with acute ischemic stroke and a transient rise in BP can be found in previously normotensive patients Citation[3]. The mechanism that causes elevation in the BP with the onset of stroke is unknown and questions remain as to appropriate management. On the other hand, BP may decline spontaneously and unpredictably, without intervening medication Citation[4].
The incorrect use of antihypertensive drugs in acute stroke may reduce pressure‐dependent cerebral perfusion to the ischemic penumbra and increase the cerebral damage Citation[5]. Conversely, post‐stroke hypertension could be deleterious and may facilitate the development of edema in the ischemic tissue, as well as hemorrhage in the ischemic penumbra Citation[6]. Studies report varying results regarding the prognostic value of high BP in acute stroke Citation[7–9]. Furthermore, little is known about the relationship between the type and site of acute stroke, the rise of BP following stroke, and the neurological outcome.
The aim of the present study was to look for existence of a relationship between the location of ischemic stroke and the pattern of variability and degree of hypertension during the first week of post‐stroke hospitalization.
Materials and methods
Patients
The Ethics Committee of the Medical Center verified that the design of the study complies with IHC‐GCP rules and it is in accordance with its requirements.
The study included clinical data of 95 consecutive patients admitted for acute stroke to our Department during 2001. Each patient was evaluated by at least one specialist in internal medicine and a neurologist. The diagnosis was based on clinical presentation and confirmed by computed tomography (CT) findings of the brain, carried out during the first 48 h after admission. Neurologists, unaware of the patient's BP, reviewed the CT scans to characterize the stroke type and its location. Ten patients (10.5%) with signs of hemorrhage at the time of the CT scan were excluded from the study. Ischemic stroke was diagnosed in the remaining 85 cases (50 males and 35 females) and were divided into three groups according to stroke location: left cerebral hemisphere – 30 patients (three with coma, 18 with hemiparesis or hemiplegia and nine with other neurological manifestations; right cerebral hemisphere – 32 patients (three with coma, 16 with hemiparesis or hemiplegia and 13 others), and non‐localized – 23 patients (two coma, 10 with hemiparesis or hemiplegia and 11 others). Demographic data and medical history were recorded, as well as whether the patient had hypertension prior to the stroke.
BP measurement
Systolic and diastolic BP was measured at the time of admission and every day at 07.00 h during the days of hospitalization. To obtain more reliable measurements, each of two successive measurements was averaged. BP was measured 2 h before administration of BP medication, the patient being in recumbent position and always with the same instrument (Collin BP 8800, Colin Corporation, Japan).
Statistical analysis
Differences in measurements between the groups were analyzed using one‐way multiple analysis of variance (MANOVA), one‐way analysis of variance (ANOVA), and the post hoc Student–Neuman–Keul (SNK) test. Differences in BP level associated with stroke localization and hypertension were examined using two‐way ANOVA (BP×hypertension as a two‐way interaction effect). Variability of systolic and diastolic BP among the seven averaged measurements was calculated using a formula for standard deviation: . BP variability was indicated by the average standard deviation for each group of patients, with a high average standard deviation indicating high BP variability. Differences in categorical data between groups were analyzed by chi‐square tests. Differences between BP profiles of the three groups were examined with ANOVA and a subtest called Profile Analysis.
Results
Background and medical history
The only significant difference in medical history or treatment between the three groups was history of tobacco smoking (); in the group with ischemic stroke in the right cerebral hemisphere, there was a higher percentage of smokers.
Table I. Demographic and medical history of study participants, number (%).
BP
MANOVA revealed no significant differences between groups in average levels of systolic and diastolic BP (mmHg) at admission: right hemisphere (147.94/76.53±20.72/13.70), left hemisphere (151.81/76.10±25.69/16.23), non‐localized (155.23/83.41±30.45/15.74). The systolic and diastolic BP measurements of patients with left cerebral hemispheric stroke were less stable and with greater variability (p<0.001; ). The standard deviation for all days differed between patients with localized and non‐localized strokes (). A significant interaction effect of hypertension history×stroke location was found for systolic BP variability (p<0.001). Among patients without history of hypertension, those with non‐localized stroke showed more stable BP (i.e. lower standard deviation; 3.08) than patients with stroke in the right (10.73) or left (18.02) hemisphere (, ). For patients with a history of hypertension, the systolic BP was higher and more stable (lower standard deviation) among patients in the non‐localized (4.46) group than in the right (12.06) or left (14.60) hemisphere group (, ).
Figure 1. Systolic blood pressure in ischemic stroke patients during the first 7 days after stroke. Patients with right hemispheric stroke showed lower systolic blood pressure than those with left or non‐localized stroke. The blood pressure of patients with left cerebral hemispheric stroke was least stable, with more extremes.
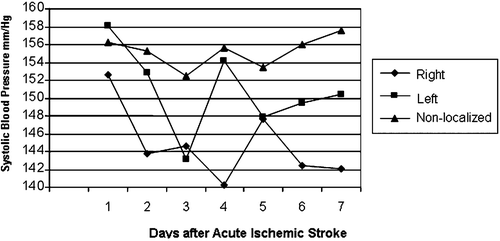
Figure 2. Systolic blood pressure in patients with no prior diagnosis of hypertension. Measurements were lowest among patients with non‐localized acute ischemic stroke. The blood pressure of patients with left cerebral hemispheric stroke was less stable and with more extremes than in other groups.
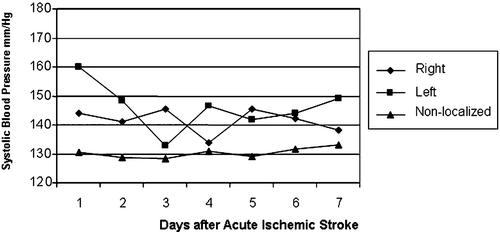
Figure 3. Systolic blood pressure patients with prior diagnosis of hypertension. Blood pressure was highest among patients with non‐localized acute ischemic stroke than in the other groups. The blood pressure of patients with left cerebral hemispheric stroke was less stable and showed more extremes.
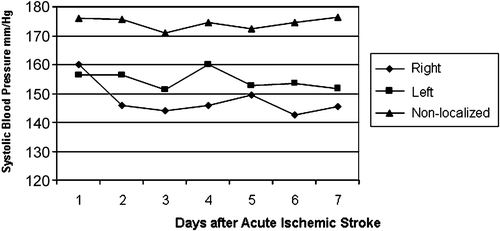
Table II. Systolic and diastolic blood pressure variability levels, mean (±SD), according to stroke localization and prior hypertension (HTN).
Mean BP profile analyses
In order to test the BP profiles of right, left and non‐localized stroke and the BP variability in hypertensive and non‐hypertensive patients during the 7 days after occurrence of stroke, three‐way ANOVA was conducted with location×hypertension history×time as independent measures. A significant interaction of time×stroke location indicated that left stroke patients had higher fluctuations in mean systolic and diastolic BP between the second and third day (p<0.05), and in systolic BP between the third and fourth day (p<0.01), while right and non‐localized stroke patients had a lower systolic BP variability on these days. Interaction of location×history of hypertension×time (three‐way interaction) indicated that left stroke non‐hypertensive patients had varying levels of mean systolic BP from the first to the second day (p<0.05) and from the third to the fourth day than those with right or non‐localized stroke. These changes between days and stroke location were lower and non‐significant among patients with a history of hypertension.
There was no relation between difference in BP variability and neurological status during admission. A detailed examination of the size and location of the strokes on CT scans did not show any statistical difference between the patients with right and left hemispheric events.
Discussion
Acute stroke is often accompanied by a transient rise in BP in both normotensive and hypertensive individuals Citation[1],Citation[3]. Although there is no data from controlled trials concerning management of hypertension in the acute phase of stroke, as many as 40% of patients remain hypertensive for about a week after acute ischemic stroke Citation[10].
An updated Cochrane survey of controlled trials suggests that the clinical outcome of acute stroke is similar whether BP is acutely lowered or left to decline spontaneously Citation[11]. Most trials covered by the Cochrane survey were small and until recently, used a calcium antagonist as active treatment. The beneficial effect of acutely lowering BP may result in a lower risk of deterioration of ischemic brain tissue Citation[4]. Spontaneous thrombolysis observed during ischemic stroke may induce hyperemia in the ischemic area and high BP could cause hemorrhagic transformation of the infarct. On the other hand, a so‐called penumbra zone of underperfused, but still viable tissue may surround the infarcted brain tissue. Acutely lowering of the BP at the onset of stroke may jeopardize the perfusion of the penumbra, leading to enlargement of the infarcted area Citation[12]. Since there is no clinical correlation between spontaneous thrombolysis and the outcome in acute ischemic stroke, it is difficult to decide to treat very high BP or not Citation[13].
Recent studies indicate that lowering systemic BP after stroke reduces the risk of recurrent stroke or vascular events Citation[14]. Other reports provide evidence that BP should not be lowered in the first week after stroke. Additional investigations indicate that elevated BP in the first few days after stroke may improve the outcome in selected patients.
Current acute ischemic stroke guidelines suggest that systolic BP up to 220 mmHg and diastolic BP up to 120 mmHg should be tolerated, except when thrombolytic therapy is indicated. In case of hemorrhagic stroke, the cutoff should be 185/110 mmHg. It is suggested that BP should be lowered carefully since it can decline spontaneously and unpredictably Citation[4].
Little is known about the relationship between the type and site of acute stroke, elevation of BP and neurological outcome. We have found in the present work a significant difference in the variability of BP measurements between patients in the three groups. Right hemispheric stroke patients tended to have lower systolic BP values than those with left hemispheric or non‐localized stroke and BP values in patients with left cerebral hemispheric stroke were more variable. Furthermore, our results indicate that changes in mean BP are differential, especially during the first 4 days after stroke and more prevalent among left stroke patients without a history of hypertension.
These findings may correlate with the notion that the ventrolateral medulla oblongata contains neurons that are the origin of tonic sympathetic discharge and they are an important center for the regulation for sympathetic and cardiovascular activities Citation[15,16]. Chemical and electrical stimulation of the ventrolateral medulla oblongata increases the BP Citation[17,18]. Jannetta & Gendel Citation[19] have described surgical patients with neurogenic hypertension, suggesting that pulsatile compression at the left side of the ventrolateral medulla oblongata may cause arterial hypertension. Later on, several clinical reports supported this concept Citation[20–25]. However, the clinical significance of neurovascular compression of the ventrolateral medulla oblongata is controversial. Kimura et al. Citation[26] have studied 69 patients under 50 years of age with ischemic stroke confirmed by magnetic resonance imaging (MRI) and found that hypertension was more frequent in young patients with neurovascular compression of the left ventrolateral medulla oblongata than those without. Seventy one per cent of the patients with neurovascular compression on the left or both sides had hypertension after an acute stroke, findings similar to those in other studies Citation[8],Citation[23],Citation[27]. In addition, neurovascular compression was more frequent in patients with lacunar stroke. Neurovascular compression of the rostral ventrolateral medulla at the left side is considered one of the causes of essential hypertension. The ventrolateral medulla regulates tonic sympathetic activity and plays a critical role in the control of arterial BP Citation[28]. Animal experiments demonstrate that acute pulsatile compression of the ventrolateral medulla increases arterial pressure by enhancing sympathetic outflow Citation[17,18]. Several investigators reported that patients with neurovascular compression show enhanced sympathetic nerve activity compared with those without neurovascular compression Citation[29–31], and clinical studies have indicated the existence of a causal relationship between hypertension and neurovascular compression on the left, but not on the right side of the ventrolateral medulla Citation[23–27],Citation[32].
We realize that our study has certain limitations due to BP measurements at a single point. However, this is a preliminary study and for future research, 24‐h monitoring would be advisable.
In conclusion, the present work indicates that the systolic BP in patients with acute ischemic stroke in the left hemisphere tends to be higher and less stable than in patients with right ischemic stroke. Prior diagnosed hypertension affects the BP profile in patients with acute but non‐localized ischemic stroke. These results contribute to the understanding of BP variability during acute ischemic stroke and hopefully, to better treatment.
References
- Semplicini A., Maresca A., Boscolo G., Sartori M., Rocchi R., Giantin V., et al. Hypertension in acute ischemic stroke: A compensatory mechanism or an additional damaging factor?. Arch Intern Med 2003; 163: 211–216
- Mancia G. Prevention and treatment of stroke in patients with hypertension. Clin Ther 2004; 26: 631–648
- Wallace J. D., Levy L. L. Blood pressure after stroke. JAMA 1981; 246: 2177–2180
- Britton M., Carlsson A., de Faire U. Blood pressure course in patients with acute stroke and matched controls. Stroke 1986; 17: 861–864
- Fischberg G. M., Lozano E., Rajamani K., Ameriso S., Fisher M. J. Stroke precipitated by moderate blood pressure reduction. J Emerg Med 2000; 19: 339–346
- Lees K. R., Dyker A. G. Blood pressure control after acute stroke. J Hypertens 1996; 14(Suppl)S35–S38
- Ahmed N., Wahlgren G. High initial blood pressure after acute stroke is associated with poor functional outcome. J Intern Med 2001; 249: 467–473
- Carlberg B., Asplund K., Hagg E. The prognostic value of admission blood pressure in patients with acute stroke. Stroke 1993; 24: 1372–1375
- Bath F. J., Bath M. W. P. What is the correct management of blood pressure in acute stroke? The blood pressure in acute stroke collaboration. Cerebrovasc Dis 1997; 7: 205–213
- Arquizan C. Hypertension in the acute phase and in the secondary prevention of stroke. Rev Prat 2004; 54: 637–640
- The Cochrane Library. Blood Pressure in Acute Stroke Collaboration (BASC). Interventions for deliberately altering blood pressure in acute stroke. Cochrane Review,. John Wiley & Sons, Chichester 2004; 1
- Olsen T. S., Larsen B., Herning M., Bech Skriver E. R., Lassen N. A. Blood flow and vascular reactivity in collaterally perfused brain tissue. Evidence of an ischemic penumbra in patients with acute stroke. Stroke 1983; 14: 332–341
- Strandgaard S. The management of elevated blood pressure in acute stroke: Preferential use of angiotensin II receptor antagonists?. J. Hypertension 2004; 22: 877–878
- Hillis A. E. Systemic blood pressure and stroke outcome and recurrence. Curr Atheroscler Rep 2004; 6: 274–280
- Callaresu F. R., Yardley C. P. Medullary basal sympathetic tone. Annu Rev Physiol 1988; 50: 511–524
- Ciriello J., Caverson M. M., Polosa C. Function of the ventrolateral medulla in the control of the circulation. Brain Res 1986; 396: 359–391
- Morimoto S., Sasaki S., Miki S., Kawa T., Itoh H., Nakata T., et al. Pulsatile compression of the rostral ventrolateral medulla in hypertension. Hypertension 1997; 29: 514–518
- Morimoto S., Sasaki S., Miki S., Kawa T., Nakamura K., Ichida T., et al. Pressor response to compression of the ventrolateral medulla mediated by glutamate receptors. Hypertension 1999; 33: 1207–1213
- Jannetta P. J., Gendel H. M. Neurovascular compression associated with essential hypertension. Neurosurgery 1978; 2: 165
- Jannetta P. J., Gendel H. M. Clinical observations on etiology of essential hypertension. Surg Forum 1979; 30: 431–432
- Fein J. M., Frishman W. Neurogenic hypertension related to vascular compression of the lateral medulla. Neurology 1980; 6: 615–622
- Jannetta P. J., Segal R., Wolfson S. K., Jr., Dujovny M., Semba A., Cook E. E. Neurogenic hypertension: Etiology and surgical treatment. II. Observations in an experimental nonhuman primate model. Ann Surg 1985; 202: 253–261
- Kleineberg B., Becker H., Gaab M. R., Naraghi R. Essential hypertension associated with neurovascular compression: Angiographic findings. Neurosurgery 1992; 30: 834–841
- Naraghi R., Gaab M. R., Walter G. F., Kleineberg B. Atrial hypertension and neurovascular compression at the ventrolateral medulla. J Neurosurg 1992; 77: 103–112
- Naraghi R., Geiger H., Crnac J., Huk W., Fahlbusch R., Engels G., et al. Posterior fossa neurovascular anomalies in essential hypertension. Lancet 1994; 334: 1466–1470
- Kimura K., Minematsu K., Yonemura K., Koga M., Yasaka M., Yamaguchi T. Hypertension and neurovascular compression of the left lateral medulla oblongata in ischemic stroke. Eur Neurol 2001; 46: 70–74
- Colon G. P., Quint D. J., Dickinson L. D., Brunberg J. A., Jamerson K. A., Hoff J. T., et al. Magnetic resonance evaluation of ventrolateral medullary compression in essential hypertension. J Neurosurgery 1998; 88: 226–231
- Granata A. R., Ruggiero D. A., Park D. H., Joh T. H., Reis D. J. Brain stem area with C1 epinephrine neurons mediates baroreflex vasodepressor responses. Am J Physiol 1985; 17: 547–567
- Morimoto S., Sasaki S., Takeda K., Furuya S., Naruse S., Matsumoto K., et al. Decreases in blood pressure and sympathetic nerve activity by microvascular decompression of the rostral ventrolateral medulla in essential hypertension. Stroke 1999; 30: 1707–1710
- Morise T., Horita M., Kitagawa I., Shinzato R., Hoshiba Y., Masuya H., et al. The potent role of increased sympathetic tone in pathogenesis of essential hypertension with neurovascular compression. J Hum Hypertens 2000; 14: 807–811
- Makino Y., Kawano Y., Okuda N., Horio T., Iwashima Y., Yamada N., et al. Autonomic function in hypertensive patients with neurovascular compression of the ventrolateral medulla oblongata. J Hypertens 1999; 17: 1257–1263
- Levy E. I., Clyde B., McLaughlin M. R., Jannetta P. J. Microvascular decompression of the lateral medulla oblongata for severe refractory neurogenic hypertension. Neurosurgery 1998; 43: 1–9