Abstract
Aims. The purpose of the study was to assess the effect of percutaneous transluminal coronary angioplasty (PTCA) on plasma N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) level in hypertensive and normotensive subjects with and without systolic left ventricular dysfunction. Methods and results. Forty patients affected by ischemic heart disease and submitted to PTCA were studied. The patients were divided into four groups: group I – 10 patients with essential arterial hypertension (HT) and normal left ventricular ejection fraction (EF); group II – 10 patients with HT and EF <55%; group III – 10 patients without HT and with normal EF; group IV – 10 patients without HT and with EF <55%. Blood samples were collected twice: 24 h before and after PTCA. The plasma NT‐proBNP concentrations increased significantly in group I (368±103 pg/ml vs 488±182 pg/ml; p<0.05), in group III (257±107 pg/ml vs 447±198 pg/ml; p<0.05), and in group IV (419±99 pg/ml vs 826±432 pg/ml; p<0.05) 24 h after PTCA. There were significant differences in the relative change in plasma NT‐proBNP concentrations between groups I and II, and between groups III and IV. Conclusions. Successful coronary angioplasty results in a rise in plasma NT‐proBNP concentration. The increase is less expressive in patients with systolic left ventricular dysfunction. The presence of hypertension does not affect NT‐proBNP concentration after PTCA.
Introduction
The role of B‐type natriuretic peptide (BNP) in the clinical expression of heart failure (HF) has been well established Citation[1,2]. Increased plasma concentrations of BNP and N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) are sensitive biochemical markers of systolic and diastolic ventricular dysfunction Citation[3–5]. Contemporary investigations have correlated concentrations of circulatory BNP Citation[6] and NT‐proBNP Citation[7] with severity of HF and it can provide incremental refinement for improving the diagnosis of HF Citation[1]. Other studies have demonstrated that myocardial ischemia is also associated with an increase in plasma concentrations of BNP and NT‐proBNP Citation[8,9]. Plasma levels of BNP and NT‐proBNP are markedly increased in patients with acute coronary syndromes and are powerful predictors of the later risk of death, onset of heart failure and new acute coronary events Citation[10–13]. Recently, it has been shown that baseline plasma NT‐proBNP levels before percutaneous transluminal coronary angioplasty (PTCA) provide important, independent prognostic information for the occurrence of death and myocardial infarction Citation[14]. Although the majority of patients with acute coronary syndromes, as well as patients with stable ischemic heart disease (IHD), undergo PTCA, plasma BNP response during an episode of myocardial ischemia induced by PTCA has not been well established. There is also uncertain whether the presence of essential arterial hypertension (HT) and impaired systolic left ventricular function modulate this response.
This study was designed to assess the effect of myocardial revascularization by means of PTCA on plasma NT‐proBNP concentration and to compare the behavior of this peptide in hypertensive and normotensive subjects with and without systolic left ventricular dysfunction.
Patients and methods
Patients
We studied 40 patients affected by IHD and submitted to PTCA. The patients were divided into four groups: group I – 10 patients with HT and normal left ventricular ejection fraction (EF); group II – 10 patients with HT and EF <55%; group III – 10 patients without HT and with normal EF; and group IV – 10 patients without HT and with EF <55%. Patients with an acute coronary syndrome, cardiomyopathy, sustained arrhythmia including atrial fibrillation, uncontrolled hypertension or secondary form of hypertension, congestive heart failure, diabetes mellitus, renal insufficiency, liver failure, chronic obstructive lung disease and apnea syndrome were excluded from the study. Hypertension was defined as systolic blood pressure ⩾140 mmHg, diastolic blood pressure ⩾90 mmHg, or use of antihypertensive drugs. Patients were on standard therapy with aspirin, clopidogrel, beta‐blocker, angiotensin‐converting enzyme inhibitor and statin. Patients with HT additionally received diuretics. The treatment was not changed before or during the study.
The study was approved by local Scientific Human Committee; written informed consent was obtained from recruited patients.
Plasma NT‐proBNP measurements
Blood samples for NT‐proBNP measurement were performed twice: 24 h before PTCA and 24 h after PTCA. Blood was collected in tubes containing Na2‐EDTA (1.5 mg/ml) with aprotinin (500 KIU/ml), placed on ice and centrifuged at 1800 rpm for 15 min. All the samples were measured 30–40 min after sampling. Plasma levels of NT‐proBNP were measured using an electrochemiluminescence immunoassay (Roche Diagnostics, Germany). This assay contains polyclonal antibodies, which recognize epitopes located in the N‐terminal part (1–76) of proBNP (77–108) and has no cross‐reactivity with atrial natriuretic peptide and C‐type natriuretic peptide. The lowest detection limit for this assay was 5 pg/ml.
Statistical analysis
The results are presented as means±standard deviation (SD). The changes in plasma NT‐proBNP concentrations within groups were analyzed using the paired t‐test, and between groups using analysis of variance (ANOVA); p‐values (two‐sided) of less than 5% were considered significant. All statistical tests were performed by using Statistica 5.1 (Statsoft Tulsa, OK, USA).
Results
Baseline characteristics of the study sample are shown in . The plasma NT‐proBNP concentrations in patients undergoing PTCA were significantly different just before the intervention between groups I and II (368±103 pg/ml vs 1481±533 pg/ml; p = 0.0001), between groups II and III (1481±533 pg/ml vs 257±107 pg/ml; p<0.0001), and between groups II and IV (1481±533 pg/ml vs 419±99 pg/ml; p = 0.0002). Twenty‐four hours after PTCA, the plasma NT‐proBNP concentration increased significantly from 368±103 pg/ml to 488±182 pg/ml (p<0.05) in group I, from 257±107 pg/ml to 447±198 pg/ml (p<0.05) in group III, and from 419±99 pg/ml to 826±432 pg/ml (p<0.05) in group IV; the rise in the plasma NT‐proBNP concentration 24 h after PTCA in group II was not significant (1481±533 pg/ml vs 1603±582 pg/ml; p>0.05; ).
Table I. Baseline patient characteristics.
Table II. Mean plasma NT‐proBNP concentrations 24 h pre‐ and post‐PTCA.
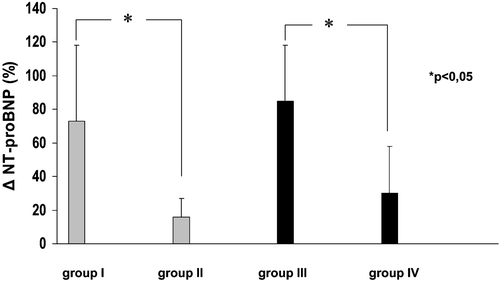
There were significant differences in the relative change in plasma NT‐proBNP concentrations calculated as a percentage of baseline values between groups I and II (73±45% vs 16±11%; p<0.05), and between groups III and IV (85±33% vs 30±28%; p<0.05) (). The relative change in plasma NT‐proBNP concentrations was similar in hypertensive and normotensive patients with normal EF (73±45% vs 85±33%; p>0.05). Similarly, the relative change in plasma NT‐proBNP concentrations was comparable between the group of patients with impaired systolic left ventricular function and HT, and the group of patients with impaired systolic left ventricular function without HT (16±11% vs 30±28%; p>0.05).
Discussion
The present study shows that plasma NT‐proBNP concentration significantly increases after PTCA. In most studies assessing the effect of PTCA on plasma BNP and NT‐proBNP concentrations, the increase in these peptides levels has been demonstrated after coronary angioplasty Citation[15–18]. The transient left ventricular dysfunction caused by reperfusion stunning may be the mechanism underlying the rise in plasma NT‐proBNP level after PTCA. During PTCA, the balloon inflation causes the coronary artery occlusion that leads to transient myocardial ischemia followed by reperfusion after the deflation of the angioplasty balloon. Despite lack of irreversible injury and despite successful reperfusion, transient regional wall motion abnormalities of heart muscle are observed Citation[19]. Mechanical abnormalities produced by ischemia and reperfusion affect both systolic and diastolic function Citation[19].
Although PTCA is a clinical situation in which stunned myocardium may occur, numerous clinical studies have shown recovery of systolic left ventricular function several minutes after reperfusion Citation[20–25]. However, there is no recovery of diastolic left ventricular function Citation[20–28]. Some groups of investigators observed relatively prolonged abnormalities in diastolic function of the left ventricle after the balloon deflation Citation[27,28].
HT, like other cardiovascular risk factors, has been reported to increase reperfusion injury Citation[29,30]. Although the exact mechanisms remain unclear, one recurring theme is that the increased oxidative stress and endothelial cell dysfunction may underlie hypertension‐mediated exacerbation of reperfusion injury Citation[30]. It might be expected that PTCA will produce greater increase in NT‐proBNP concentration in patients with HT. However, increased plasma NT‐proBNP levels reflect not only ventricular dysfunction, but may also result from myocardial ischemia. Myocardial ischemia per se results in increase in plasma NT‐proBNP concentration Citation[31]. Experimental and clinical studies have demonstrated that short or long periods of myocardial ischemia are associated with the rapid release of BNP and induction of the peptide synthesis de novoCitation[15–17],Citation[32,33]. In a rat model of coronary artery ligation, plasma BNP concentration in the left ventricle increased about twofold as early as 12 h after acute myocardial infarction (AMI) and fivefold after 24 h compared with the control Citation[34]. In clinical studies, both BNP and NT‐proBNP levels are raised to more than 100 times the reference levels within a few hours from the onset of AMI Citation[35,36].
Although severe ischemia leading to AMI is always associated with ventricular dysfunction, which may be the principal stimulus for BNP synthesis and release, there is evidence that milder ischemic episodes, in the absence of substantial ventricular dysfunction, are associated with elevation of circulating BNP levels Citation[31]. Talwar et al. compared circulating concentrations of NT‐proBNP in patients with stable and unstable angina pectoris Citation[8]. Plasma NT‐proBNP levels were raised in unstable angina in comparison with the age‐matched control without IHD and patients with stable angina. Clinical observations that BNP is released rapidly in response to even brief episodes of myocardial ischemia are supported by experimental studies Citation[32,33]. D'Souza measured BNP concentration in the coronary effluent after 2, 5 and 20 min of global normothermic ischemia followed by reperfusion in perfused rat hearts Citation[33]. BNP levels were found to correlate with the duration of ischemia. Since neither 2‐min nor 5‐min ischemia was associated with a rise in end‐diastolic pressure, the study has shown that ischemia per se, rather than changes in wall‐stress secondary to ischemia, promoted BNP release.
It is postulated that the rapid release of BNP from ventricular myocardium and the early activation of the natriuretic peptide receptor may be an important autocrine/paracrine response to ischemia. BNP was found to attenuate tissue susceptibility to ischemic injury Citation[31],Citation[33]. BNP limits the extent of tissue damage during ischemia and reperfusion. BNP administration to isolated perfused rat hearts before and during left main coronary artery occlusion resulted in limitation of infarct size in a concentration‐dependent fashion Citation[33].
We have demonstrated that the relative change in the plasma NT‐proBNP concentration after PTCA was significantly less in the groups of patients with impaired systolic left ventricular function comparing with those with normal EF, independently on the presence of HT. We hypothesize that ventricular dysfunction is the stimulus for synthesis and release of BNP, which outweighs the influence of other modulating factors, including myocardial ischemia. The plasma NT‐proBNP concentrations at baseline were significantly higher in the groups of patients with impaired systolic left ventricular function. Therefore, the increase in the plasma NT‐proBNP concentration after PTCA in these groups of patients should be much higher than in the groups of patients with normal EF to produce the similar relative change of the peptide levels. Myocardial ischemia itself is not such a powerful determinant that could induce such a high increase in plasma NT‐proBNP levels.
In conclusion, the present study shows that successful myocardial revascularization by means of PTCA results in rise in plasma NT‐proBNP concentration. The increase is less expressive in patients with impaired systolic left ventricular function. The presence of hypertensive disease does not affect NT‐proBNP concentration after PTCA.
References
- Kelly R., Struthers A. D. Are natriuretic peptides clinically useful as markers of heart failure?. Ann Clin Biochem 2001; 38: 94–102
- Adams K. F., Jr., Mathur V. S., Gheorghiade M. B‐type natriuretic peptide from bench to bedside. Am Heart J 2003; 145(Suppl)34–46
- McDonagh T. A., Robb S. D., Murdoch D. R., Morton J. J., Ford I., Morrison C. E., et al. Biochemical detection of left‐ventricular systolic dysfunction. Lancet 1998; 351: 9–13
- Cowie M. R., Struthers A. D., Wood D. A., Coats A. J., Thompson S. G., Poole‐Wilson P. A., et al. Value of natriuretic peptides in assessment of patients with possible new heart failure in primary care. Lancet 1997; 350: 1349–1353
- Lubien E., DeMaria A., Krishnaswamy P., Clopton P., Koon J., Kazanegra R., et al. Utility of B‐natriuretic peptide in detecting diastolic dysfunction: Comparison with Doppler velocity recordings. Circulation 2002; 105: 595–601
- Wieczorek S. J., Bailly K. R., Thomas P., Wu A. H. B., Hager D., Ferrier A., et al. Clinical evaluation of the Triage B‐type natriuretic peptide assay for the point of care testing of patients with congestive heart failure. Clin Chem 2000; 46: A77
- Hunt P. J., Richards A. M., Nicholls M. G., Yandle T. G., Doughty R. N., Espiner E. A. Immunoreactive amino‐terminal pro‐brain natriuretic peptide (NT‐proBNP): A new marker of cardiac impairment. Clin Endocrinol 1997; 47: 287–296
- Talwar S., Squire I. B., Downie P. F., Davies J. E., Ng L. L. Plasma N terminal pro‐brain natriuretic peptide and cardiotrophin 1 are raised in unstable angina. Heart 2000; 84: 421–424
- Bibbins‐Domingo K., Ansari M., Schiller N. B., Massie B., Whooley M. A. B‐type natriuretic peptide and ischemia in patients with stable coronary disease: Data from the Heart and Soul study. Circulation 2003; 108: 2987–2992
- de Lemos J. A., Morrow D. A., Bentley J. H., Omland T., Sabatine M. S., McCabe C. H., et al. The prognostic value of B‐type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med 2001; 345: 1014–1021
- Horio T., Shimada K., Kohno M., Yoshimura T., Kawarabayashi T., Yasunari K., et al. Serial changes in atrial and brain natriuretic peptides in patients with acute myocardial infarction treated with early coronary angioplasty. Am Heart J 1993; 126: 293–299
- Jiang C. Y., Li N., Wang J. A. Use of B‐type natriuretic peptide in evaluation of early percutaneous coronary intervention in patients with acute coronary syndromes. Chin Med J 2004; 117: 1130–1134
- Katayama T., Nakashima H., Yonekura T., Honda Y., Suzuki S., Yano K. Clinical significance of acute‐phase brain natriuretic peptide in acute myocardial infarction treated with direct coronary angioplasty. J Cardiol 2003; 42: 195–200
- de Winter R. J., Stroobants A., Koch K. T., Bax M., Schotborgh C. E., Mulder K. J., et al. Plasma N‐terminal pro‐B‐type natriuretic peptide for prediction of death or nonfatal myocardial infarction following percutaneous coronary intervention. Am J Cardiol 2004; 94: 1481–1485
- Kyriakides Z. S., Markianos M., Michalis L., Antoniadis A., Nikolaou N. I., Kremastinos D. T. Brain natriuretic peptide increases acutely and much more prominently than atrial natriuretic peptide during coronary angioplasty. Clin Cardiol 2000; 23: 285–288
- Tateishi J., Masutani M., Ohyanagi M., Iwasaki T. Transient increase in plasma brain (B‐type) natriuretic peptide after percutaneous transluminal coronary angioplasty. Clin Cardiol 2000; 23: 776–780
- Goetze J. P., Christoffersen C., Perko M., Arendrup H., Rehfeld J. F., Kastrup J., et al. Increased cardiac BNP expression associated with myocardial ischemia. FASEB J 2003; 17: 1105–1107
- Palazzuoli A., Carrera A., Calabria P., Pastore M., Quatrini I., Vecchiato L., et al. Brain natriuretic peptide levels during cardiac reperfusion: Comparison between percutaneous coronary angioplasty and aorto‐coronaric bypass. Clin Chim Acta 2004; 342: 87–92
- Braunwald E. Stunning of the myocardium: An update. Cardiovasc Drugs Ther 1991; 5: 849–851
- Alam M., Khaja F., Brymer J., Marzelli M., Goldstein S. Echocardiographic evaluation of left ventricular function during coronary artery angioplasty. Am J Cardiol 1986; 57: 20–25
- Doorey A. J., Mehmel H. C., Schwarz F. X., Kubler W. Amelioration by nitroglycerin of left ventricular ischemia induced by percutaneous transluminal coronary angioplasty: Assessment by hemodynamic variables and left ventriculography. J Am Coll Cardiol 1985; 6: 267–274
- Serruys P. W., Wijns W., van den Brand M., Meij S., Slager C., Schuurbiers J. C., et al. Left ventricular performance, regional blood flow, wall motion and lactate metabolism during transluminal angioplasty. Circulation 1984; 70: 25–36
- Hauser A. M., Gangadharan V., Ramos R. G., Gordon S., Timmis G. C. Sequence of mechanical, electrocardiographic and clinical effects of repeated coronary artery occlusion in human beings: Echocardiographic observations during coronary angioplasty. J Am Coll Cardiol 1985; 5: 193–197
- Visser C. A., David G. K., Kan G., Romijn K. H., Meltzer R. S., Koolen J. J., et al. Two‐dimensional echocardiography during percutaneous transluminal coronary angioplasty. Am Heart J 1986; 111: 1035–1041
- Wohlgelernter D., Cleman M., Highman H. A., Fetterman R. C., Duncan J. S., Zaret B. L., et al. Regional myocardial dysfunction during coronary angioplasty: Evaluation by two‐dimensional echocardiography and 12 lead electrocardiography. J Am Coll Cardiol 1986; 7: 1245–1254
- Wijns W., Serruys P. W., Slager C. J., Grimm J., Krayenbuehl H. P., Hugenholtz P. G., et al. Effect of coronary occlusion during percutaneous transluminal angioplasty in humans on left ventricular chamber stiffness and regional diastolic pressure‐radius relations. J Am Coll Cardiol 1986; 7: 455–463
- Carlson E. B., Hinohara T., Morris K. G. Recovery of systolic and diastolic left ventricular function after a 60‐second coronary arterial occlusion during percutaneous transluminal coronary angioplasty for angina pectoris. Am J Cardiol 1987; 60: 460–466
- Kloner R. A., Jennings R. B. Consequences of brief ischemia: Stunning, preconditioning, and their clinical implications: Part 1. Circulation 2001; 104: 2981–2989
- Verma S., Fedak P. W., Weisel R. D., Butany J., Rao V., Maitland A., et al. Fundamentals of reperfusion injury for the clinical cardiologist. Circulation 2002; 105: 2332–2336
- Granger D. N. Ischemia‐reperfusion: Mechanisms of microvascular dysfunction and the influence of risk factors for cardiovascular disease. Microcirculation 1999; 6: 167–178
- Baxter G. F. Natriuretic peptides and myocardial ischaemia. Basic Res Cardiol 2004; 99: 90–93
- Toth M., Vuorinen K. H., Vuolteenaho O., Hassinen I. E., Uusimaa P. A., Leppaluoto J., et al. Hypoxia stimulates release of ANP and BNP from perfused rat ventricular myocardium. Am J Physiol 1994; 266: H1572–H1580
- D'Souza S. P., Yellon D. M., Martin C., Schulz R., Heusch G., Onody A., et al. B‐type natriuretic peptide limits infarct size in rat isolated hearts via KATP channel opening. Am J Physiol Heart Circ Physiol 2003; 284: H1592–H1600
- Hama N., Itoh H., Shirakami G., Nakagawa O., Suga S., Ogawa Y., et al. Rapid ventricular induction of brain natriuretic peptide gene expression in experimental acute myocardial infarction. Circulation 1995; 92: 1558–1564
- Morita E., Yasue H., Yoshimura M., Ogawa H., Jougasaki M., Matsumura T., et al. Increased plasma levels of brain natriuretic peptide in patients with acute myocardial infarction. Circulation 1993; 88: 82–91
- Mukoyama M., Nakao K., Obata K., Jougasaki M., Yoshimura M., Morita E., et al. Augmented secretion of brain natriuretic peptide in acute myocardial infarction. Biochem Biophys Res Commun 1991; 180: 431–436