Abstract
Cardiovascular and renal diseases share many of the same risk factors. In fact, renal failure is usually accompanied by an increased global cardiovascular risk. Thus, preservation of kidney function might simultaneously protect the heart and the brain and, conversely, addressing cardiovascular risk factors might safeguard the kidney. This review considers the evidence supporting this approach, focusing on the protective effect of blood‐pressure lowering and the ancillary actions of antihypertensive agents on renal protection. We review recent evidence on renal protection in individuals with and without diabetes, and the importance of offering a high standard of care also to those with the metabolic syndrome or prediabetes in order to prevent initial forms of renal, and as a consequence, cardiovascular damage. Intervention may be appropriate even in individuals with high‐normal blood pressure, if they already have early renal and/or cardiovascular risk markers. As a consequence of these insights, thresholds for starting antihypertensive therapy are gradually falling, whereas awareness of the need for an early intervention in patients at high risk of developing renal damage and simultaneously cardiovascular disease is growing.
Introduction
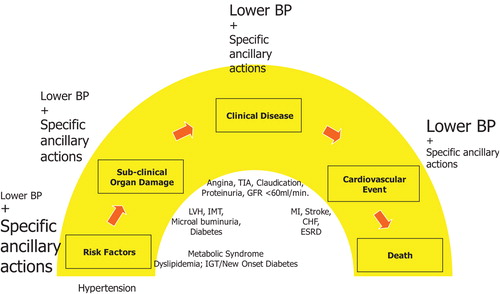
The number of patients needing dialysis for end‐stage renal disease (ESRD) and the costs of their treatment have increased dramatically over the last 15 years and continue to rise. Although this increase is partly due to the recent decline in cardiovascular (CV) mortality and hence to more people surviving enough as to develop ESRD, most deaths in patients presenting with early stages of chronic kidney disease (CKD) as well as those with ESRD are due to CV events Citation[1]. This is hardly surprising, since the kidney is an integral part of the CV system, and the development of renal and CV disease (CVD) can be regarded as a continuum, starting with factors that damage both the renal and the systemic vasculature while allowing, in most cases, a rise in blood pressure (BP) and then progressing, first to sub‐clinical organ damage, then to clinical manifestations of disease, and finally to end‐stage disease (Figure ) Citation[2–4]. Consistent with this view is the observation that both CVDs and kidney diseases share many of the same risk factors, with diabetes and hypertension predominating Citation[1], Citation[5].
Figure 1 The cardiovascular continuum in hypertension and the relative preventive effect of blood pressure lowering and the ancillary actions of antihypertensive agents. In the therapeutic continuum, the size of the letters indicates the relative and changing importance of the various approaches at various stages of the cardiovascular disease continuum. IGT, impaired glucose tolerance; LVH, left ventricular hypertrophy; IMT, carotid intima‐media thickening; TIA, transient ischaemic attack; MI, myocardial infarction; CHF, congestive heart failure; ESRD, end‐stage renal disease. Adapted from references 2 and 3.
Although diabetes is a major risk factor for CKD and CVD, both diabetic and non‐diabetic nephropathies carry a similar risk for the incidence of new CV events Citation[6]. In fact, the risk of any CV event doubles as the estimated glomerular filtration rate (eGFR) falls from ⩾60 ml/min/1.73 m2 to 30–44 ml/min/1.73 m2Citation[6]. Consequently, preventing the decline in kidney function, regardless of its cause, might be protective for other parts of the cardiovasculature. By the same token, addressing CV risk should help to safeguard the kidney. We review here recent evidence on renal protection that could support this approach, focusing on early and adequate interventions on CV risk factors that counteract with both CV and renal disease progression.
Recent evidence in established renal disease
Non‐diabetic renal disease
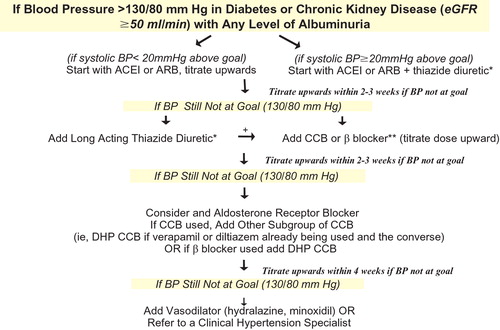
Research has identified a number of risk factors and/or markers that are common to both CVD and CKD (Table ), many of which are modifiable by lifestyle changes and/or pharmacological intervention Citation[1], Citation[5]. An adequate diet with a moderate to low sodium intake must always be contemplated and will contribute to both facilitate BP control as well as to slow down the velocity of progression of the components depicted in Figure . In subjects with CKD, antihypertensive therapy should aim for a target of ⩽130/80 mmHg first using angiotensin‐converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs) to inhibit the renin–angiotensin system (RAS) and adding diuretics and other agents as needed Citation[7], Citation[8]. Figure contains the algorithm proposed by the National Kidney Foundation Citation[9] for simultaneous renal and CV protection in patients with CKD depending with eGFR values equal to or above 50 ml/min/1.73 m2. The type of the diuretic used, loop diuretic, is the only difference when eGFR is <50 ml/min/1.73 m2. The BP goal should be even lower if proteinuria above 1 g/day is present. ACE inhibitors and ARBs are particularly indicated when proteinuria is present in both diabetic Citation[10], Citation[11] and non‐diabetic Citation[12] nephropathies. For those patients presenting with renal failure in the absence of albuminuria, the administration of a drug suppressing the RAS must also be contemplated because these individuals have to be considered high‐risk patients Citation[1] but also because some evidence to be confirmed shows that renal protection is better with long‐term therapy with an ACE inhibitor Citation[13].
Table I. Risk factors/markers for both cardiovascular disease and chronic kidney disease.
Figure 2 Algorithm for cardiovascular and renal protection in patients with chronic kidney disease and estimated glomerular filtration rate⩾50 ml/min. *Exercise caution over the use of high doses of diuretics. If a beta‐blocker is prescribed, then it should be combined with a DHP CCB. **Carvedilol is preferred because of specific data in renal impairment and outcomes. Other beta‐blockers are not excluded, there are no data to support the use of atenolol in such patients and only limited data to support metoprolol, which is also poorly tolerated. eGFR, estimated glomerular filtration rate; BP, blood pressure; ARB, angiotensin II receptor blocker; ACEI, angiotensin‐converting enzyme inhibitor; CCB, calcium‐channel blocker; DHP CCB, dihydropyridine calcium‐channel blocker.
The reduction in proteinuria is associated with a slower progression of both renal and CVD Citation[14]. There may also be an argument for using a calcium‐channel blocker (CCB) in combination with either an ACE inhibitor or an ARB Citation[15]; the main reason should be the additive effect on BP control. As shown in the REIN‐2 trial, adding felodipine to ramipril lowered BP although it was not associated with a further reduction in proteinuria in patients with non‐diabetic chronic nephropathy Citation[16].
Another interesting study is the COOPERATE trial Citation[17] that provides further insights into blood‐pressure‐independent effects of RAS inhibition in non‐diabetic renal disease. This study investigated the ARB losartan and the ACE inhibitor trandolapril used either alone or in combination, titrated or combined to achieve similar levels of BP control. Dual inhibition of the RAS with the combination further reduced both proteinuria and the percentage of patients with progressive kidney disease in comparison with either agent used alone. An ambulatory BP monitoring (ABPM) substudy confirmed that 24‐h BP levels were identical among treatment groups Citation[18]. These findings have been recently expanded in a meta‐analysis showing a greater reduction in proteinuria in patients treated with the combination of ACE inhibitors and ARB Citation[19], indicating that patients with proteinuric renal disease should receive either one or even both classes of agents regardless of BP levels and, indeed, even if these levels are normal. Preliminary data also suggest that the addition of selective aldosterone antagonists could be of additional benefit in proteinuric CKD patients Citation[20].
ACE inhibitors have also been shown to reduce CV risk when compared to placebo in high‐risk patients Citation[21] and preliminary data indicate that this class of drugs could be particularly effective to reduce CV events in patients with CKD that represent a situation of high added risk Citation[22], Citation[23]. At present, no studies are available comparing ARB to placebo in such high‐risk patients. On the other hand, both ACE inhibitors and ARB have been shown to prevent the development of new onset diabetes in most comparative trials with other antihypertensive drug classes Citation[24].
Diabetic renal disease
Various recently published studies, have proven the value of ARB for renal protection in diabetic nephropathy Citation[11], Citation[25], Citation[26] confirming and extending previous data obtained with ACE inhibitors Citation[10]. A direct comparison of an ACE inhibitor (enalapril) and an ARB (telmisartan) on renal outcomes in type 2 diabetic patients, most of them microalbuminuric, showed a similar renal protective capacity of the two drugs during the 5 years of follow‐up Citation[27]. Interestingly, at the end of the study, the levels of albuminuria did not differ from those detected at baseline. This could be an argument in favour of considering higher doses or combinations of the RAS blockers during long‐term follow‐up in order to maintain a constant drop in albuminuria when this marker is present at baseline. Another study of great interest is the BENEDICT (BErgamo NEphrologic DIabetes Complications Trial Citation[28])) designed to assess whether ACE inhibitors and non‐dihydropyridinic CCBs, alone or in combination, prevent microalbuminuria in patients with hypertension, type 2 diabetes and normal urinary albumin excretion. A total of 1204 subjects were randomly assigned to receive at least 3 years treatment with trandolapril, alone or in combination with verapamil, verapamil alone, or placebo. The primary outcome was reached in 5.7%, 6.0%, 11.9% and 10.0% of patients, respectively. Thus, the incidence of microalbuminuria in the group receiving the ACE inhibitor either alone or combined was approximately half that observed in the other two groups. Similar studies are ongoing with ARB. Indeed, the ROADMAP trial Citation[29] is aimed to assess the effect of olmesartan as compared to placebo in the primary prevention of microalbuminuria in type 2 diabetics. Furthermore, the DIRECT study Citation[30], the primary end‐point of which is the prevention of diabetic retinopathy, has as a secondary end‐point the development of de novo microalbuminuria in type 1 and type 2 diabetic patients.
Metabolic syndrome, prediabetes and diabetes as risk factors for CVD and renal disease
The clustering of CV risk factors accompanying high BP is the main characteristic of the metabolic syndrome. Although definitions vary somewhat, there is a general agreement that the metabolic syndrome predicts the development of both type 2 diabetes and coronary heart disease Citation[31]. Since most individuals with the syndrome are insulin resistant and obese, initial therapy comprises caloric restriction and increased physical activity. Conventional CV risk factors such as dyslipidaemia and hypertension often coexist with the presence of metabolic syndrome and should also receive appropriate pharmacological treatment; preliminary data indicate that control of BP and hypercholesterolaemia does not prevent CV risk as well as it does in those patients without metabolic syndrome, and thus the residual risk of these patients is still high.
The relevance of metabolic syndrome in the development of CV events and death has been confirmed in many studies. In the Framingham Heart Study, metabolic syndrome was present in more than one‐third of the CV events and death Citation[32]. Similarly, there is a close relationship between the presence of microalbuminuria and of CKD and the number of altered components of the metabolic syndrome according to the definition proposed by the National Cholesterol Education Program‐Adult Treatment Panel III Citation[33], Citation[34]. Thus, metabolic syndrome is viewed as a prediabetic situation and seems to be related to the presence of CKD, which in turn could precede the development of diabetes mellitus. It has also been shown to participate actively in the development of overt arterial hypertension in individuals previously diagnosed as presenting with normal or high‐normal BP Citation[35].
The goals of identifying and treating the prediabetic patient are devoted to prevent the development of diabetes and to minimize other CV risk factors Citation[36], Citation[37]. Prediabetes not only is a strong risk factor for diabetes Citation[38] but also for CVD Citation[39], Citation[40], which often appears years before type 2 diabetes develops Citation[41]. There are, however, some important differences between prediabetes and diabetes, which determine the extent of care needed Citation[36]. Thus, unlike individuals with diabetes, those with prediabetes are not at increased risk of infection, microvascular complications or hypoglycaemia. They therefore do not need HbA1c measurements, eye examinations or foot examinations. Close monitoring either by the individuals themselves or by their physicians is less important and, at present, BP and lipid goals are less rigorous than for diabetics Citation[36]. However, as previously commented for metabolic syndrome, a relevant percentage of these patients present with microalbuminuria and the finding of a decreased eGFR is not infrequent in these patients indicating that the above‐mentioned relation between prediabetes and CKD is of great importance when CV and renal prognosis are considered.
The definition of prediabetes has been classically based on the presence of an impaired fasting serum glucose (currently >5.55 mmol/l) or carbohydrate intolerance (serum glucose between 7.8 and 11.0 mmol/l, 2 h after an oral glucose tolerance test). Actually, the presence of abdominal obesity and associated alterations included in the definition of metabolic syndrome could be more appropriate. These subjects should be screened at least once a year for the presence of diabetes Citation[42]. As mentioned above, antihypertensive treatment with ACE inhibitors or ARB prevents diabetes development in treated hypertensives, whereas diuretics and beta‐blockers tend to increase its incidence Citation[24].
In this sense, the Study of Trandolapril/Verapamil SR and Insulin Resistance (STAR) has provided complementary evidence. The study compared an ACE inhibitor and a CCB fixed‐dose combination, versus a thiazide diuretic and an ARB fixed‐dose combination, on glucose tolerance in 240 patients with metabolic syndrome. The primary endpoint was the change in 2‐h post‐prandial glucose using oral glucose tolerance testing after a 1‐year follow‐up. Interestingly, the ARB was not able completely to counteract the deleterious effect of the thiazide diuretic on post‐prandial plasma glucose, which resulted in an increase at the end of the study. Conversely, the combination of the ACE inhibitor and the CCB had no effect on this parameter Citation[4].
The diabetic patient
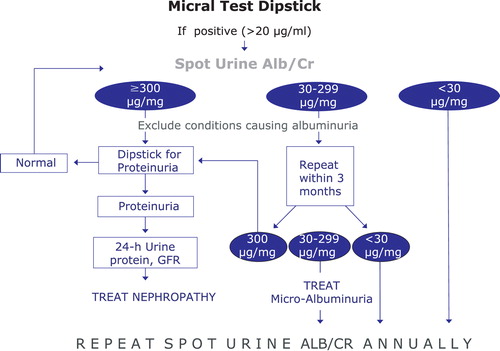
Diabetic patients need regular close monitoring because they are at high risk of both macrovascular and microvascular complications, which tight glycaemic, BP and lipid control can prevent Citation[44]. This is crucial because diabetes is reaching epidemic proportions worldwide, and the disease and its complications have an enormous socio‐economic impact Citation[45]. Early screening of urinary albumin excretion and estimation of GFR is mandatory in every diabetic patient and these determinations must be repeated at least once yearly either to discover new alterations or to control those already existing (Figure ). An integral intervention on CV risk with strict lifestyle changes, physical activity, glycaemic and BP control, suppression of renin–angiotensin–aldosterone system, statins and other hypolipidaemic drugs, and aspirin are required particularly in those patients with any degree of CKD Citation[1], Citation[5].
The relevance of BP control as compared to suppression of the RAS in the prevention of CKD
Despite the substantial epidemiological evidence of a continuous relationship between CV and renal complications and systolic BP to levels as low as 120 mmHg Citation[46], Citation[47], most intervention trials have had higher threshold values for recruitment: ⩾160/90 mmHg for essential hypertension and, for isolated systolic hypertension, ⩾160 mmHg systolic with diastolic BP <90 or 95 mmHg. All trials have achieved significant reductions in CVD regardless of the class of agent used Citation[48], Citation[49]. However, a recently published meta‐analysis casts doubts about the beneficial effects of renin–angiotensin suppression when plotted versus BP control for renal protection Citation[50]. It is true that we still miss the trial in which a strict BP control is attained to see whether in this case the protection does not require suppression of the RAS. However, with the available evidence, this type of therapy is required if we desire to get the maximal protection for the kidney. Even more, because of the increase in CV risk, it should be needed for CV protection Citation[21].
Conclusion: Integrating renal and CV protection
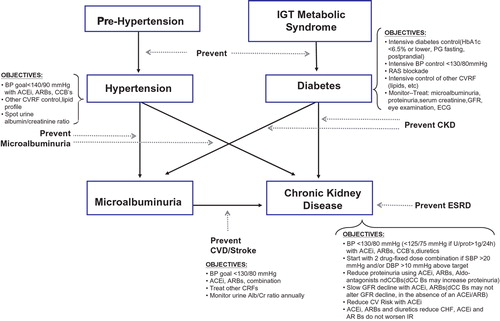
Figure depicts and summarizes the possible simultaneous prevention of CV and renal disease. Simultaneous prevention or retardation in the development of arterial hypertension and diabetes must be accompanied by the simultaneous prevention of their early complications, in particular microalbuminuria. Later on, integral CV and renal prevention are totally similar because renal and CV protection run in parallel.
Figure 4 The relationship between hypertension, diabetes and chronic kidney disease and treatment objectives. IGT, impaired glucose tolerance; PG, plasma glucose; BP, blood pressure; RAS, renin–angiotensin system; CVRF, cardiovascular risk factor; GFR, glomerular filtration rate; ECG, electrocardiogram; CKD, chronic kidney disease; ESRD, end‐stage renal disease; ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CCB, calcium‐channel blocker; CVD, cardiovascular disease.
New guidelines start to contemplate an integral approach to global CV risk, considering situations precluding the development of diabetes and of renal disease such as prediabetes. A nice example is the recently published guidelines on diabetes, prediabetes and CVD Citation[51].
References
- Brosius F. C., Hostetter T. H., Kelepouris E., Mitsnefes M. M., Moe S. M., Moore M. A., et al. Detection of chronic kidney disease in patients with or at increased risk of cardiovascular disease: A science advisory from the American Heart Association Kidney and Cardiovascular Disease Council; the Councils on High Blood Pressure Research, Cardiovascular Disease in the Young, and Epidemiology and Prevention; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: Developed in Collaboration With the National Kidney Foundation. Circulation 2006; 114: 1083–1087
- Dzau V., Braunwald E. Resolved and unresolved issues in the prevention and treatment of coronary artery disease: A workshop consensus statement. Am Heart J 1991; 121: 1244–1263
- Zanchetti A. Evidence‐based medicine in hypertension: What type of evidence?. J Hypertens 2005; 23: 1113–1120
- Segura J., Garcia‐Donaire J. A., Praga M., Ruilope L. M. Chronic kidney disease as a situation of high added risk in hypertensive patients. J Am Soc Nephrol 2006; 17 Suppl 2: S136–S140
- Ruilope L. M., van Veldhuisen D. J., Ritz E., Luscher T. F. Renal function: The Cinderella of cardiovascular risk profile. J Am Coll Cardiol 2001; 38: 1782–1787
- Go A. S., Chertow G. M., Fan D., McCulloch C. E., Hsu C. Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296–1305
- Chobanian A. V., Bakris G. L., Black H. L., Cushman W. C., Green L. A., Izzo J. L., et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure. Hypertension 2003; 42: 1206–1252
- Guidelines Committee. 2003 European Society of Hypertension–European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens 2003; 21: 1011–1053
- K‐DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 2004; 43: S1–S290
- Lewis E. J., Hunsicker L. G., Bain R. P., Rohde R. D. The effect of angiotensin‐converting‐enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 1993; 329: 1456–1462
- Brenner B. M., Cooper M. E., de Zeeuw D., Keane W. F., Mitch W. E., Parving H. H., et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–869
- Wright J. T., Bakris G., Greene T., Agodoa L. Y., Appel L. J., Charleston J., et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: Results from the AASK trial. JAMA 2002; 288: 2421–2431
- Segura J., Campo C., Rodicio J. L., Ruilope L. M. ACE inhibitors and appearance of renal events in hypertensive nephrosclerosis. Hypertension 2001; 38: 645–649
- De Zeeuw D., Remuzzi G., Parving H. H., Keane W. F., Zhang Z., Shahinfar S., et al. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation 2004; 110: 921–927
- Bakris G. L. Implications of albuminuria on kidney disease progression. J Clin Hypertens (Greenwich) 2004; 6 Suppl 3: 18–22
- Ruggenenti P., Perna A., Loriga G., Ganeva M., Ene‐Iordache B., Turturro M., et al. Blood‐pressure control for renoprotection in patients with non‐diabetic chronic renal disease (REIN‐2): Multicentre, randomised controlled trial. Lancet 2005; 365: 939–946
- Nakao N., Yoshimura A., Morita H., Takada M., Kayano T., Ideura T. Combination treatment of angiotensin‐II receptor blocker and angiotensin‐converting‐enzyme inhibitor in non‐diabetic renal disease (COOPERATE): A randomised controlled trial. Lancet 2003; 361: 117–124
- Nakao N., Seno H., Kasuga H., Toriyama T., Kawahara H., Fukagawa M. Effects of combination treatment with losartan and trandolapril on office and ambulatory blood pressures in non‐diabetic renal disease: A COOPERATE‐ABP substudy. Am J Nephrol 2004; 24: 543–548
- MacKinnon M., Shurraw S., Akbari A., Knoll G. A., Jaffey J., Clark H. D. Combination therapy with an angiotensin receptor blocker and an ACE inhibitor in proteinuric renal disease: A systematic review of the efficacy and safety data. Am J Kidney Dis 2006; 48: 8–20
- Epstein M. Aldosterone blockade: An emerging strategy for abrogating progressive renal disease. Am J Med 2006; 119: 912–919
- Dagenais G. R., Poque J., Fox K., Simoons M. L., Yusuf S. Angiotensin‐converting‐enzyme inhibitors in stable vascular disease without left ventricular systolic dysfunction or heart failure: A combined analysis of three trials. Lancet 2006; 368: 581–588
- Mann J. F., Gerstein H. C., Poque J., Bosch J., Yusuf S. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: The HOPE randomized trial. Ann Intern Med 2001; 134: 629–636
- Segura J., Campo C., Gil P., Roldan C., Vigil L., Rodicio J. L., et al. Development of chronic kidney disease and cardiovascular prognosis in essential hypertensive patients. J Am Soc Nephrol 2004; 15: 1616–1622
- Mancia G., Grassi G., Zanchetti A. New onset diabetes and antihypertensive drugs. J Hypertens 2006; 24: 3–10
- Parving H. H., Lehnert H., Brochner‐Mortensen J., Gomis R., Andersen S., Arner P. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 2001; 345: 870–878
- Lewis E. J., Hunsicker L. G., Clarke W. R., Berl T., Pohl M. A., Lewis J. B., et al. Renoprotective effect of the angiotensin‐receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345: 851–860
- Barnett A. H., Bain S. C., Bouter P., Karlberg B., Madsbad S., Jervell J., , for the Diabetics Exposed to Telmisartan and Enalapril Study Group, et al. Angiotensin‐receptor blockade versus converting‐enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med 2004; 351: 1952–1961
- Ruggenenti P., Fassi A., Ilieva A. P., Bruno S., Iliev I. P., Brusegan V., , for the Bergamo Nephrologic Diabetes Complications Trial (BENEDICT) Investigators, et al. Preventing microalbuminuria in type 2 diabetes. N Engl J Med 2004; 351: 1941–1951
- Haller H., Viberti G. C., Mimran A., Remuzzi G., Rabelink A. J., Ritz E., et al. Preventing microalbuminuria in patients with diabetes: Rationale and design of the Randomised Olmesartan and Diabetes Microalbuminuria Prevention (ROADMAP) study. J Hypertens 2006; 24: 403–408
- Chatuverdi N., Sjoelie A. K., Svensson A., for the DIRECT Programme Study Group. The DIabetic Retinopathy Candesartan Trials (DIRECT) Programme, rationale and study design. J Renin Angiotensin Aldosterone Syst 2002; 3: 255–261
- Grundy S. M., Cleeman J. I., Daniels S. R., Donato K. A., Eckel R. H., Franklin B. A., et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005; 112: 2735–2752
- Wilson P. W., D'Agostino R. B., Parise H., Sullivan L., Meigs J. B. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 2005; 112: 3066–3072
- Whaley‐Connell A., Sowers J. R. Chronic kidney disease and the cardiometabolic syndrome. J Clin Hypertens (Greenwich) 2006; 8: 546–548
- Chen J., Muntner P., Hamm L. L., Jones D. W., Batuman V., Fonseca V., et al. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med 2004; 140: 167–174
- Cordero A., Laclaustra M., Leon M., Grima A., Casanovas J. A., Luengo E., et al. Prehypertension is associated with insulin resistance state and not with an initial renal function impairment. A Metabolic Syndrome in Active Subjects in Spain (MESYAS) Registry substudy. Am J Hypertens 2006; 19: 189–196
- American Diabetes Association, National Institute of Diabetes and Digestive and Kidney Diseases. Prevention or delay of type 2 diabetes. Diabetes Care 2004; 27 Suppl 1: S47–S54
- Haffner S. M. The prediabetic problem: Development of non‐insulin‐dependent diabetes mellitus and related abnormalities. J Diabetes Complications 1997; 11: 69–76
- de Vegt F., Dekker J. M., Jager A., Hienkens E., Kostense P. J., Stehouwer C. D., et al. Relation of impaired fasting and postload glucose with incident type 2 diabetes in a Dutch population: The Hoorn Study. JAMA 2001; 285: 2109–2113
- The DECODE Study Group: Glucose tolerance and cardiovascular mortality: Comparison of fasting and 2‐hour diagnostic criteria. Arch Intern Med 2001; 161: 397–405
- Saydah S. H., Loria C. M., Eberhardt M. S., Brancati F. L. Subclinical states of glucose intolerance and risk of death in the US. Diabetes Care 2001; 24: 447–453
- Manley S. M., Meyer L. C., Neil H. A. W., Ross I. S., Turner R. C., Holman R. R. Complications in newly diagnosed type 2 diabetic patients and their association with different clinical and biochemical risk factors. UKPDS 6. Diabetes Res 1990; 13: 1–11
- American Diabetes Association. Screening for type‐2 diabetes. Diabetes Care 2004; 27(Suppl 1): S11–S14
- Bakris G., Molitch M., Sowers J., Hewkin A., Fakouhi K., Bacher P. Differences in glucose tolerance between antihypertensive combination drugs in metabolic syndrome patients. Results of STAR. J Clin Hypertens (Greenwich) 2006; 8(suppl A): A177 [Abstract]
- Gaede P., Vedel P., Larsen N., Jensen G. V., Parving H. H., Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003; 348: 383–393
- King H., Aubert R. E., Herman W. H. Global burden of diabetes, 1995–2025: Prevalence, numerical estimates, and projections. Diabetes Care 1998; 21: 1414–1431
- Lewington S., Clarke R., Qizilbash N., Peto R., Collins R., for the Prospective Studies Collaboration. Age‐specific relevance of usual blood pressure to vascular mortality: A meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360: 1903–1913
- Tozawa M., Iseki K., Iseki C., Kinjo K., Ikemiya Y., Takishita S. Blood pressure predicts risk of developing end‐stage renal disease in men and women. Hypertension 2003; 41: 1341–1345
- Blood Pressure Lowering Treatment Trialists' Collaboration. Effects of different blood‐pressure‐lowering regimens on major cardiovascular events: Results of prospectively‐designed overviews of randomised trials. Lancet 2003; 362: 1527–1535
- Turnbull F., Neal B., Algert C., Chalmers J., Chapman N., Cutler J., , for the Blood Pressure Lowering Treatment Trialists' Collaboration, et al. Effects of different blood pressure‐lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: Results of prospectively designed overviews of randomized trials. Arch Intern Med 2005; 165: 1410–1419
- Casas J. P., Chua W., Loukogeorgakis S., Vallance P., Smeeth L., Hingorani A. D., et al. Effect of inhibitors of the renin–angiotensin system and other antihypertensive drugs on renal outcomes: Systematic review and meta‐analysis. Lancet 2005; 366: 2026–2033
- The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). Guidelines on diabetes, prediabetes, and cardiovascular disease: Executive summary. Eur Heart J 2007; 28: 88–136