Abstract
Background/aims. Aortic stenosis (AS) and hypertension are associated with cardiac hypertrophy and aortic dilatation. The effect of their coincidence on the ascending aortic dimensions has not yet been evaluated, and therefore was the aim of our study. Methods. We performed cross‐sectional analysis of history, clinical, angiographic and echocardiographic data of consecutive patients evaluated before surgery for non‐rheumatic AS. Results. The study sample included 225 patients (age 68±9 years, 60% males), with mean transaortic gradient of 55±17 mmHg. Hypertension was present in 153 (68%) patients. The hypertensives had more severe dyspnea (NYHA class 2.2±0.9 vs 1.9±0.9, p = 0.05) and higher prevalence of coronary artery disease (57% vs 33%, p = 0.001), but did not differ from the normotensives in the ascending aortic dimensions, the left ventricular mass, ejection fraction and remodeling patterns. Wider ascending aortic dimensions were independently associated with bicuspid aortic valve (p<0.001), and with maximal gradient in those with tricuspid aortic valve. Vasodilators were used in 84 (54%) hypertensives. Conclusion. We found hypertension in 68% of patients with severe AS. Bicuspid aortic valve and stenosis severity were independent predictors of ascending aortic dimensions, but not the history of hypertension and blood pressure.
Introduction
Calcific aortic stenosis (AS) is an atherosclerosis related disease found in 2.5% of patients older than 65 years, and the most frequent cause of valve surgery in developed countries Citation[1]. It may be associated with aortic dilatation requiring treatment decision at the time of surgery Citation[2]. Intrinsic aortic wall structure abnormalities associated with bicuspid aortic valve and severity of regurgitation are considered the risk factors of aortic dilatation Citation[3], Citation[4], while independent association between aortic dimensions and stenosis severity has not been observed Citation[5].
A wide range of hypertension prevalence, 30–55%, has been reported in AS Citation[6], Citation[7]. Aorta and the proximal arteries stiffen with age. The descending aorta dilates with age and hypertension Citation[8], Citation[9]. The sum effect of hypertension and AS on the ascending aorta as a possible target organ for both diseases has not yet been evaluated.
We aimed to assess the association of hypertension with the ascending aortic dimensions and to assess hypertension prevalence and treatment in patients with non‐rheumatic severe AS.
Patients and methods
The study population
We performed a retrospective analysis of prospectively collected data of consecutive patients with AS, who underwent evaluation before aortic valve replacement with or without revascularization at the Ist Department of Medicine, Charles University of Prague, School of Medicine Hospital in Pilsen. The AS was defined by the mean transaortic gradient of ⩾30 mmHg or aortic valve area<1 cm2/m2. Between January 2002 and June 2006, 367 patients were referred for the examination. We excluded patients with rheumatic heart disease (defined by fusion of commissures between the valve cusps plus rheumatic mitral valve disease), prior aortic valve replacement, congenital heart disease except for bicuspid aortic valve, moderate to severe aortic regurgitation (>2/4), Marfan syndrome, infectious endocarditis, hypertrophic obstructive cardiomyopathy, acute coronary syndrome, and those in the dialysis program. According to these criteria, 267 patients were eligible. Excluding those with incomplete data, 225 patients were finally included into the analysis. The study followed the principles established in the Declaration of Helsinki.
Medical history, clinical data and laboratory assessment
We obtained history of major cardiovascular risk factors, type and dosage of medication from the hospital records. Hypertension was defined by the documented history of systolic blood pressure (BP)>140 mmHg or diastolic BP>90 mmHg or antihypertensive medication. Casual BP was measured before the echocardiographic examination after 5 min in the sitting position. Height and weight were also measured at the time of the echocardiographic examination.
Fasting blood samples were taken in all patients for basic laboratory assessments, including lipid, glucose and creatinine levels. Creatinine clearance was calculated using the Cockroft–Gault formula.
Echocardiography
We performed standard echocardiographic examination in all, and multiplane transesophageal echocardiography in 80% of patients (System FiVE, Vivid Five, GE Vingmed, Horten, Norway; iE33, Philips, Andover, MA, USA). The examinations were randomly performed by two echocardiographers with known interobserver variability of 5%. The measurements were made offline from the videotapes and data from three beats were averaged. Mean and peak transaortic gradients were calculated by the modified Bernoulli equation at the time of examination. Aortic regurgitation was evaluated according to the American Society of Echocardiography (ASE) criteria Citation[10].
The left ventricular (LV) and aortic dimensions were measured following the standard ASE criteria Citation[11] from the two‐dimensional long axis parasternal view by the leading edge to leading edge method. The aorta was measured at the level of the aortic annulus, sinuses of Valsalva, sinotubular junction and proximal ascending aorta, 1–1.5 cm from the sinotubular junction Citation[12]. The aortic dimensions were indexed by the body surface area.
The ejection fraction was assessed from the M mode using the Teichholz formula, or from the two‐dimensional four‐chamber view using the Simpson's formula. The LV mass was calculated by the ASE recommended formula Citation[13]. Relative wall thickness (RWT) was calculated by the formula RWT = (2×PWTd)/LVIDd, where PWTd was the posterior diastolic wall thickness and LVIDd the LV inner diastolic dimension.
Normal geometry was identified if the RWT⩽0.42 and the LV mass index (LVMI)<95 g/m2 in females or <115 g/m2 for males. Concentric remodeling was identified if RWT>0.42 and LVMI<95 g/m2 in females or <115 g/m2 in males. Concentric hypertrophy was identified if RWT>0.42 and LVMI⩾95 g/m2 in females or ⩾115 g/m2 in males, and excentric hypertrophy was identified if RWT⩽0.42 and LVMI⩾95 g/m2 in females or ⩾115 g/m2 for males Citation[13].
Bicuspid aortic valve was defined by the presence of two commissures and two aortic cusps Citation[14] on the parasternal or midesophageal short axis view. The echocardiographic findings were checked with surgical findings and consensus between echocardiography and surgery was reached in all cases.
Coronary angiography
Coronary angiography was performed in all patients older than 40 years, and in younger only in the presence of major cardiovascular risk factors. Significant coronary artery disease (CAD) was defined by more than 50% diameter stenosis.
Statistics
We used the Statgraphics Centurion Version XV (Statpoint Inc., Herndon, VA, USA) for statistical evaluation. The continuous variables are shown as mean and standard deviation. The categorical variables are shown as number and percentage. The continuous variables were compared by the t‐test or by the Mann–Whitney U‐test as appropriate, the binomical variables by the chi‐quadrate test. We included age, sex, systolic and diastolic BP, pulse pressure, transaortic gradients, history of hypertension and valve morphology as independent variables, and aortic dimensions and LV parameters as dependent variables. We used the simple regression with the best alternative fit transformation and the multiple regression method; p<0.05 was considered statistically significant.
Results
Basic clinical characteristics
Our study sample included 225 patients. We found arterial hypertension in 153 (68%), hyperlipidemia in 151 (67%), diabetes in 65 (29%), and current or past smoking in 83 (37%) patients. Basic characteristics of the study sample and comparison between hypertensives and normotensives with AS are shown in the Table . Dyspnea or angina were the common symptoms present in 205 (91%) patients, while syncope was scarce, manifested in 29 (13%) patients only. Both dyspnea and angina were more severe in the hypertensives than normotensives with AS. Hypertension was associated with higher prevalence of significant CAD.
Table I. Comparison of basic patient characteristics between hypertensives and normotensives with aortic stenosis.
As expected, the patients with bicuspid aortic valve were younger than those with tricuspid aortic valve (62±10 vs 70±8 years, p<0.001), but did not differ significantly in the prevalence of hypertension and in AS severity (data not shown).
Echocardiographic findings
The comparison of echocardiographic findings between normotensives and hypertensives with AS is shown in the Table . We did not find significant differences in the echocardiographic LV characteristics and distribution of the remodeling patterns between normotensives and hypertensives.
Table II. Comparison of echocardiographic characteristics between hypertensives and normotensives with aortic stenosis.
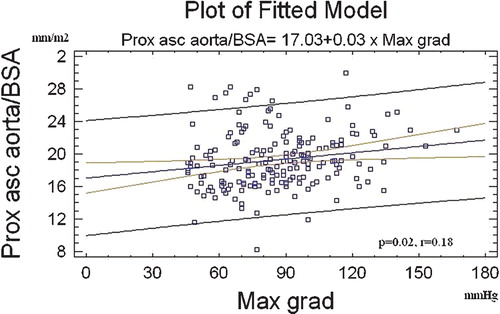
The ascending aorta was at all levels wider in patients with bicuspid than with tricuspid aortic valve (Figure ), but did not differ between hypertensives and normotensives. We found a relatively weak but significant association of the proximal ascending aortic dimension with the maximal transaortic gradient (Figure ). However, only presence of the bicuspid aortic valve was a significant independent predictor of the proximal ascending aortic diameter (Table ).
Table III. Bicuspid aortic valve as an independent predictor of the proximal ascending aorta diameter in patients with aortic stenosis.
Figure 1 Comparison of the ascending aorta diameters between patients with bicuspid and tricuspid aortic valve. BAV, bicuspid aortic valve; TAV, tricuspid aortic valve; BSA, body surface area.
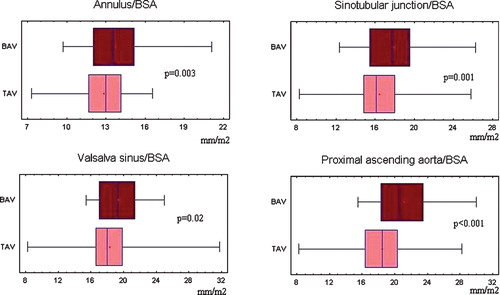
Figure 2 Correlation between the proximal ascending aorta diameter and the maximal transaortic gradient. Regression line, the 95% confidence and prediction limit lines are shown. Prox asc aorta/BSA, proximal ascending aortic dimension indexed by body surface area; Max grad, maximal gradient.
Furthermore, we divided the study sample by valve morphology and analysed each group separately. In 162 patients with tricuspid aortic valve, only maximal transaortic gradient was independently associated with the proximal ascending aorta diameter (Table ). In 63 patients with bicuspid aortic valve, we did not find independent association between proximal ascending aortic diameter and hypertension, age, pulse pressure, maximal transaortic gradient or degree of aortic regurgitation (data not shown).
Table IV. Maximal transaortic gradient as an independent predictor of the proximal ascending aorta diameter in patients with tricuspid aortic valve stenosis.
Antihypertensive medication
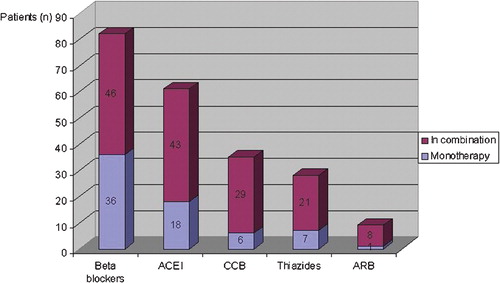
At the time of examination, out of the 153 hypertensives with AS 133 patients (87%) were on medical treatment, and of those one half needed more than one antihypertensive agent. The overview of medication is shown in the Figure . Vasodilating agents (angiotensin converting enzyme inhibitors, angiotensin II receptor blockers or calcium channel blockers) were given alone or in combination to 83 (54%) hypertensives.
Figure 3 Overview of antihypertensive medication in 133 patients with severe symptomatic AS associated with hypertension. Of 153 patients with AS associated with hypertension, 133(87%) were on medical treatment at the time of examination. The vertical bars show the percentage of hypertensives with AS treated by each class of drugs. The blue segment of the bar shows the percentage of patients treated by each drug class used as monotherapy, and the purple segment shows the percentage of patients treated by each drug class used in combination therapy. ACEI, angiotensin converting enzyme inhibitors; CCB, calcium‐channel blockers; ARB, angiotensin II receptor blockers.
Discussion
We found hypertension in two‐thirds of patients with non‐rheumatic severe calcific AS. This is a higher prevalence than reported previously Citation[6], Citation[7]; however it is similar to the general population of comparable age Citation[15]. The hypertensives were older, had more severe symptomatology, higher prevalence of CAD and higher pulse pressure than normotensives with AS.
To our knowledge, the present study is the first one that compared ascending aortic dimensions between normotensive and hypertensive patients with AS. In our study sample, wider aorta was independently associated with the presence of bicuspid aortic valve. In the subset of patients with tricuspid aortic valve wider aorta was independently associated with the maximal transaortic gradient. However, we did not find association between ascending aortic dimensions and history of hypertension, BP level or pulse pressure.
Due to selection criteria, the aortic regurgitation was mostly none or mild in our study sample, which explains why it was not a dominant factor for aortic diameters. In patients with bicuspid aortic valve, aortic wall tissue abnormalities similar to Marfan syndrome Citation[3] have been described that may contribute to aortic dilatation not primarily driven by altered hemodynamics. Though not previously observed in moderate AS Citation[5], the direct effect of the high‐velocity jet might also contribute to wider ascending aorta in those with severe tricuspid aortic valve stenosis. Our results implicate that possible wall tissue abnormalities associated with bicuspid aortic valve or the effect of the stenotic jet may be more important predictors of ascending aorta dimensions, than the afterload increase due to hypertension.
Furthermore, the LVMI and the distribution of remodeling patterns assessed according to current criteria did not differ between hypertensive and normotensive patients with AS. This confirms previous findings in patients with severe AS Citation[6]; however, it is somewhat striking, given the big systolic BP difference between hypertensives and normotensives in AS. It may implicate severe AS as a more important component of the afterload increase than hypertension, although it is known that the degree of hypertrophy is only poorly related to the severity of outflow obstruction caused by AS Citation[6], Citation[7], Citation[16], and the LVMI was not associated with aortic gradients in our study. Second, long‐term drug treatment given to the majority of hypertensives may have also modified hemodynamics and anatomy of the left heart in our patients. Further, the patients may differ in the diastolic function, and detailed analysis using tissue Doppler imaging would be necessary.
Finally, most hypertensive patients still required medication at the time of preoperative evaluation, and vasodilators were used in more than one half of them. Frequent use of angiotensin‐converting enzyme inhibitors in AS has been observed recently Citation[17]. Hypertension diagnosis and treatment choice might have preceded the diagnosis of AS, nevertheless, our data may suggest good tolerance of vasodilators even in hemodynamically severe AS.
The limitations of our study originate from its retrospective character. Only pulse pressure, the simplest measure of aortic stiffness, was available in our study and more data on duration and severity of hypertension would be of interest. Tissue Doppler indices were not available for all patients and thus not analysed. Furthermore, we are aware that the cause‐and‐effect relationship cannot be assessed from a cross‐sectional analysis.
Conclusion
To summarize, arterial hypertension was a common finding in our patients with severe symptomatic non‐rheumatic AS evaluated for valve surgery. Wider aortic dimensions were associated with bicuspid aortic valve, and with AS severity in those with tricuspid aortic valve, but not with hypertension or BP. The hypertensives with AS had significantly higher systolic and pulse BP, without difference in AS severity, left ventricular mass and systolic function indices. Though no recommendations for the treatment of hypertension may be drawn from our analysis, it shows that vasodilators are commonly used and well tolerated in the therapy of hypertension even in severe AS. We may speculate that especially blockers of the renin–angiotensin system may have beneficial effect on the left ventricular remodeling and prevention of hypertrophy even in severe AS. Prospective study is needed to test this hypothesis.
Acknowledgements
This study was supported by the grant NR/8306‐5 from the Internal Grant Agency, Ministry of Health, Prague, Czech Republic and by the cardiovascular research project of the Charles University of Prague, Czech Republic, no. MSM0021620817, entitled “The invasive approach to myocardial salvage and regeneration”.
References
- Stewart B. F., Siscovick D., Lind B. K., Gardin J. M., Gottdiener J. S., Smith V. E., et al. Clinical factors associated with calcific aortic disease: Cardiovascular Health Study. J Am Coll Cardiol 1997; 29: 630–634
- Crawford M. H., Roldan C. A. Prevalence of aortic root dilatation and small aortic roots in valvular aortic stenosis. Am J Cardiol 2001; 87: 1311–1313
- Fedak P. W. M., de Sa M. P. L., Verma S., Nili N., Kazemian P., Butany J., et al. Vascular matrix remodeling in patients with bicuspid aortic valve malformations. Implications for aortic dilatation. J Thorac Cardiovasc Surg 2003; 126: 797–805
- Novaro G. M., Tiong I. Y., Pearce G. L., Grimm R. A., Smedira N., Griffin B. P. Features and predictors of ascending aortic dilatation in association with a congenital bicuspid aortic valve. Am J Cardiol 2003; 92: 99–101
- Linhartová K., Beránek V., Šefrna F., Štěrbáková G., Pešková M. Aortic stenosis severity is not a risk factor of poststenotic dilatation of the ascending aorta. Circ J 2007; 71: 84–88
- Antonini‐Canterin F., Huang G., Cervesato E., Faggiano P., Pavan D., Piazza R., et al. Symptomatic aortic stenosis: Does systemic hypertension play an additional role?. Hypertension 2003; 41: 1268–1272
- Peltier M., Trojette F., Sarano M. E., Grigioni F., Slama M. A., Tribouilloy C. M. Relation between cardiovascular risk factors and nonrheumatic severe calcific aortic stenosis among patients with three‐cuspid aortic valve. Am J Cardiol 2003; 91: 97–99
- O'Rourke M. F., Nichols W. W. Aortic diameter, aortic stiffness, and wave reflection increase with age and systolic hypertension. Hypertension 2005; 45([part 2])652–658
- Bella J. N., Wachtell K., Boman K., Palmieri V., Papademetriou, Gerdts E., , for the LIFE Study Investigators, et al. Relation of left ventricular geometry and function to aortic root dilatation in patients with systemic hypertension and left ventricular hypertrophy [The LIFE Study]. Am J Cardiol 2002; 89: 337–340
- Zoghbi W. A., Enriquez‐Sarano M., Foster E., Grayburn P. A., Kraft C. D., Levine R. A., et al. Recommendations for evaluation of the severity of native valvular regurgitation with two‐dimensional and Doppler echocardiography. J Am Soc Echocardiogr 2003; 16: 777–802
- Schiller N. B., Shah P. M., Crawford M., De Maria A., Devereux R., Feigenbaum H., et al. Recommendations for quantitation of the left ventricle by two‐dimensional echocardiography: American Society of Echocardiography committee on standards, subcommittee on quantitation of two‐dimensional echocardiograms. J Am Soc Echocardiogr 1989; 2: 358–367
- Roman M. J., Devereux R. B., Kramer‐Fox R., O'Loughlin J. Two‐dimensional echocardiographic aortic root dimensions in normal children and adults. Am J Cardiol 1989; 64: 507–512
- Lang R. M., Bierig M., Devereux R. B., Flachskampf F. A., Foster E., Pelikka P. A., et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005; 18: 1440–1463
- Brandenburg RO J. r., Tajik A. J., Edwards W. D., Reeder G. S., Shub C., Seward J. B. Accuracy of 2‐dimensional echocardiographic diagnosis of congenitally bicuspid aortic valve: Echocardiographic‐anatomic correlation in 115 patients. Am J Cardiol 1983; 51: 1469–1473
- Burt V. L., Whelton P., Roccella E. J., Brown C., Cutler J. A., Higgins M., et al. Prevalence of hypertension in the US adult population. Results from the Third National Heart and Nutrition Examination Survey, 1988–1991. Hypertension 1995; 25: 305–313
- Kostkiewicz M., Tracz W., Olszowska M., Podolec P., Drop D. Left ventricular geometry and function in patients with aortic stenosis: Gender differences. Int J Cardiol 1999; 71: 57–61
- Rosenhek R., Rader F., Loho N., Gabriel H., Heger M., Klaar U., et al. Statins but not angiotensin‐converting enzyme inhibitors delay progression of aortic stenosis. Circulation 2004; 110: 1291–1295