Abstract
Hypertension has been associated with changes in endothelial function in both large muscular arteries and small resistance arteries. We evaluated the relationship between blood flow velocity and dilatation of the brachial artery following transient forearm ischemia and acetylcholine‐induced relaxation in subcutaneous small arteries and the influence of antihypertensive therapy on both in patients with essential hypertension. Thirty‐one previously untreated hypertensive patients were randomized in a double‐blind fashion to treatment with either the angiotensin‐converting enzyme (ACE) inhibitor perindopril or the beta‐blocker atenolol and compared with 17 healthy normotensive controls. Before and after 1 year of treatment, while still on active medication, flow‐mediated dilatation (FMD) was measured in the brachial artery using ultrasound while relaxation to acetylcholine in small arteries was tested in vitro in a myograph. FMD correlated inversely to resting brachial artery diameter (r = −0.38, p<0.05). FMD corrected for resting diameter (FMDcorr) was lower in patients (3.0±0.2%) compared with controls (4.2±0.3%, p<0.01). In both patients and controls, FMDcorr was related to flow velocity in a non‐linear way with FMDcorr reaching a maximum despite increasing flow velocities, and in the patients, FMDcorr was only reduced at high flow velocities. Furthermore, patients had a reduced acetylcholine‐induced relaxation in small arteries (p = 0.04). Perindopril and atenolol reduced blood pressure to similar levels and both drugs improved FMDcorr to a similar degree without any effects on relaxation to acetylcholine in small arteries. The present study demonstrates the role of correcting for differences in baseline diameter during measurements of FMD and a non‐linear relationship between flow velocity and FMD in the brachial artery. Furthermore, the results suggest different effects of antihypertensive treatment on endothelial function in large and small arteries.
Introduction
Hypertension is associated with increased risk of ischemic heart disease Citation[1], and accelerated coronary artery atherosclerosis may be among the contributing factors to this. Endothelial dysfunction is an established early marker of vascular disease Citation[2], Citation[3] and treatment regimens that not only reduce blood pressure (BP) but also improve endothelial function may be of advantage.
Endothelial function can be examined non‐invasively by ultrasound‐based measurements of brachial artery vasodilatory response to increasing blood flow reflecting shear stress on the vascular wall. Despite the extensive use of measurements of brachial artery flow‐mediated dilatation (FMD), only few studies have dealt with the methodological aspects of this technique. The degree of dilatation depends on the magnitude of the stimulus, i.e. reactive hyperemia. A linear relation between blood flow and FMD has been suggested in some studies Citation[4], Citation[5], whereas others find a non‐linear relation Citation[6], Citation[7] with attenuation of dilatation at higher flow rates. Furthermore, irrespective of the blood flow obtained, the basal myogenic tone of the artery influences FMD with less dilatation in arteries with larger baseline diameter Citation[8–10]. Thus, both blood flow velocity as well as baseline diameter must be taken into consideration when evaluating FMD. However, in patients with hypertension, these problems have only been sparsely investigated.
Another approach to evaluate endothelial function is pharmacologically induced vasodilatation with acetylcholine. This method can be applied in vivo by infusion of acetylcholine in the brachial artery with assessment of forearm blood flow as a measure of the vasodilatory capacity of the vascular bed. Similar information can be obtained in vitro by acetylcholine relaxation of isolated small arteries mounted in a vessel myograph. Thus, brachial artery FMD and acetylcholine relaxation describe conductance and resistance artery endothelial function, respectively. However, no investigations have examined the effect of antihypertensive treatment on both large and small arteries in the same patient population.
The aim of the present study was therefore twofold: first to describe the relation between blood flow velocity and brachial artery FMD and second to compare the effect of antihypertensive therapy with an angiotensin‐converting enzyme (ACE) inhibitor and a beta‐blocker on brachial artery as well as small artery endothelium‐mediated relaxation.
Methods
Patients and control persons
Previously untreated hypertensive patients were recruited from general practitioners and the outpatient clinic. Subjects could be included when sitting diastolic BP was 100–120 mmHg, measured three times with a mercury sphygmomanometer, on two or three occasions during an observation period of 2–6 weeks Citation[11]. Healthy persons were recruited through advertisement at the local blood bank and required to a sitting BP below 140/90 mmHg. Patients and controls underwent a clinical examination and blood analysis. None of the patients had symptoms or signs of ischemic heart disease or secondary hypertension. The study was approved by the local ethics committee and participants signed an approved consent form before entering the study.
Treatment protocol
After inclusion, the patients entered a period of 3–4 weeks with placebo treatment, during which the brachial artery measurements and subcutaneous biopsy were performed (see below). Patients were then randomly assigned to treatment with either perindopril or atenolol in a double‐blind fashion. Randomization was balanced to ensure an equal gender and age distribution in the two groups. Dosage was adjusted to achieve an office diastolic BP⩽90 mmHg. Patients started with either 4 mg perindopril or 50 mg atenolol once daily. If diastolic BP was>90 mmHg after 1 month, the dose was doubled. If necessary 2.5 or 5 mg of bendroflumethiazide was added to achieve the target BP. After 1 year of treatment, and still on active medication, the brachial artery measurements and subcutaneous biopsy were repeated. The control persons underwent one brachial artery scan and one subcutaneous biopsy.
Brachial artery diameter and blood flow velocity
Changes in right brachial artery diameter in response to reactive hyperemia were measured using high‐resolution ultrasound (Acuson 128XP/10 with a 7.0‐MHz linear‐array transducer, Mountain View, California) as previously described Citation[12–14]. After being placed in the supine position, BP was measured before commencing the scanning procedure. The artery was scanned in longitudinal sections 20–50 mm above the elbow. Depth and gain settings were optimized to identify the lumen‐to‐vessel wall interface and were kept constant during each study. After a baseline scan, a BP cuff, placed around the thickest part of the forearm, was inflated to>250 mmHg. After 4.5 min, the cuff was released and the artery was scanned from 30 s before to 90 s after cuff deflation. A second baseline scan was recorded 10 min later. Finally, nitroglycerin (NTG, 400‐µg spray) was administered sublingually and after 3 min, the artery was scanned again. All scans were recorded on super‐VHS tapes and arterial diameters were measured directly from the tape. The internal diameter was measured from the anterior to the posterior interface between the media and the adventitia. An average diameter was calculated from four cardiac cycles incident with the R wave on the electrocardiogram. Brachial artery blood flow velocity was measured by pulsed Doppler at baseline and immediately after cuff deflation, and determined as the peak velocity obtained during the first two or three cardiac cycles. All scans were performed by a single person blinded to patient treatment. The scans were read by a single observer blinded to the identity of the subject, the timing of investigation (pre‐ or during treatment) as well as to the phase of the scanning protocol (resting scan, reactive hyperemia scan or NTG scan).
Small artery relaxation
Small subcutaneous biopsies were obtained from the gluteal area as previously described Citation[15]. Small arteries, with an estimated diameter of 100–300 µm, were dissected from the biopsy and two artery segments were mounted on thin steel wires as ring preparations in a myograph Citation[15]. The passive tension–lumen relation was determined by stepwise stretching the artery, and the diameter the vessel would have had in vivo when relaxed and under a transmural pressure of 100 mmHg was determined. Thereafter the diameter was set to 90% of this value, as the vessels are known to develop maximal active tension at this diameter Citation[15].
To test tissue viability, all arteries were subjected to 5 µmol/l noradrenaline for 2 min. If active pressure exceeded 10 kPa, the arteries were considered viable and used for further experiments. Hereafter the arteries were contracted with noradrenaline (0.5–2 µmol/l), resulting in a contraction level of 80–100% of the initial response to 5 µmol/l noradrenaline, and a concentration–response curve for acetylcholine (1 nmol/l to 10 µmol/l) was constructed.
Calculations and statistical analysis
All data are expressed as mean±SEM. FMD corrected for resting diameter (FMDcorr) was calculated from the equation obtained from the linear correlation analysis between resting brachial artery diameter and FMD. Relaxation of isolated small arteries is expressed as percentage of the noradrenaline‐induced vasoconstriction level. The normotensive controls were compared with the pretreatment values of the whole group of patients using an unpaired t‐test for brachial artery data and a two‐way analysis of variance (ANOVA) for small artery data. The effect of treatment on FMDcorr in each group was tested with a paired t‐test, while differences in the effect of treatments were tested with an unpaired t‐test. The level of significance in all tests was set as p<0.05.
Results
Table describes the baseline clinical characteristics of the hypertensive patients and the normotensive control persons included in the study. BP reduction was achieved in the perindopril group with 4 mg (n = 2), 8 mg (n = 5) or 8 mg+bendroflumethiazide (n = 8) and in the atenolol group with 50 mg (n = 9), 100 mg (n = 1) or 100 mg+bendroflumethiazide (n = 5). There were no differences in BP reduction between perindopril (26±3/17±2 mmHg)‐ and atenolol (27±3/20±2 mmHg)‐treated patients. Heart rate was not influenced by perindopril (76±1 beats/min), but as expected was reduced by atenolol (58±2 beats/min).
Table I. Clinical characteristics of the patients before treatment and control persons (mean±SEM).
FMD in the brachial artery
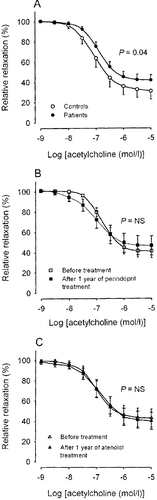
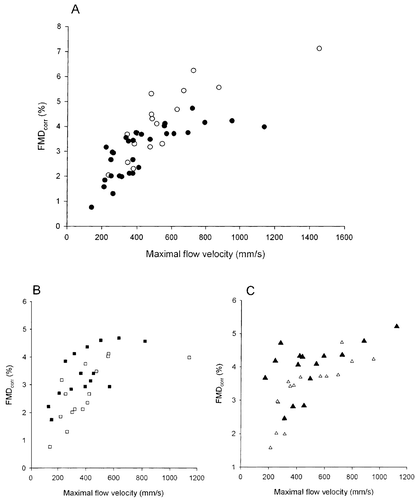
All 32 patients had successful brachial artery scans at baseline while 31 were scanned after 1 year of treatment. All 17 controls had successful scans. Mean values of brachial artery diameter and blood flow velocities are listed in Table . In untreated patients, there was a significant inverse linear correlation between resting artery diameter and FMD (y = −1.36x+8.92, r = −0.38, p<0.05), while this also was the tendency in the smaller group of controls (y = −2.51x+14.6, r = −0.41, p = 0.09). After 1 year of treatment, FMD still tended to correlate with artery diameter (y = −1.19x+8.88, r = −0.30, p = 0.11). FMDcorr was calculated based on these equations for linear regression analysis. FMDcorr was smaller in untreated patients compared with control persons (3.0±0.2% vs 4.2±0.3, p<0.01). However, for low values of hyperemic flow velocity, there was no difference between patients and controls in the relation between flow velocity and FMDcorr but as flow velocity increased, FMDcorr was progressively reduced in the patients (Figure ). FMDcorr significantly increased after 1 year with both perindopril (from 2.7±0.3% to 3.4±0.2%, p<0.01, Figure ) as well as atenolol (from 3.3±0.2 to 4.0±0.2, p<0.01, Figure ) with no difference between the two treatments. The improvement in FMDcorr was seen at both low and higher levels of hyperemic flow velocity (Figure ).
Table II. Brachial artery dimensions and flow velocities (mean±SEM).
Figure 1 The relation between maximal blood flow velocity and flow‐mediated dilatation (FMD) corrected for differences in resting diameter (FMDcorr). (A) All untreated patients with hypertension (•) and normotensive control persons (○); (B) patients before (□) and after (▪) 1 year of perindopril treatment; (C) patients before (Δ) and after (▴) 1 year of atenolol treatment.
Also, NTG‐induced dilatation of the brachial artery was dependent on the resting diameter in untreated patients (r = 0.55, p<0.01) and tended to be so in the control persons as well (r = 0.35, p = 0.20). NTG‐induced dilatation corrected for differences in resting diameters was lower in patients (13.6±0.6%) than control persons (17.4±0.6%, p<0.01). NTG‐induced dilatation slightly increased in perindopril‐treated patients (1.5±0.6%, p<0.05), whereas no change was seen during atenolol treatment (0.3±0.6%, p = NS).
Small artery relaxation
Twenty‐nine patients had a successful biopsy at baseline and 27 had a successful biopsy after 1 year of treatment, whereas 16 of the controls had a successful biopsy. The mean lumen diameter of arteries from controls (269±20 µm) was significantly higher than the lumen diameter of arteries from the hypertensive patients (221±8 µm, p<0.05). The lumen diameter in the perindopril group was 199±8 µm before and 231±8 µm after 1 year of treatment (n = 13, p = NS), whereas the diameter of the arteries in the atenolol group was 241±14 µm before and 261±22 µm after 1 year of treatment (n = 14, p = NS). There were no differences in the preconstriction levels achieved with noradrenaline between the groups. Relaxation to acetylcholine was marginally more pronounced in controls compared with the total group of hypertensive patients (p = 0.04, Figure ). However, neither perindopril nor atenolol had any influence on acetylcholine‐induced relaxation (Figure ).
Discussion
A main novel finding of the present study is that FMDcorr of the brachial artery is dependent on blood flow velocity in a non‐linear fashion and is impaired only at high hyperemic flow velocities in hypertensive patients. Furthermore, we found that FMDcorr improved by antihypertensive treatment without a concomitant improvement in small artery endothelial function.
Flow‐mediated vasodilatation
The increase in flow velocity, provoked by transient forearm ischemia, induces an increased shear stress upon the intimal surface of the brachial artery and stimulates the release of vasodilating mediators from the endothelium. In large muscular arteries, NO is a main contributor to endothelium‐mediated dilatation, as inhibitors of NO synthase almost block this response Citation[16], Citation[17]. Also flow‐induced dilatation of small arteries is predominantly dependent on NO as demonstrated in rat Citation[18] and human Citation[19] small arteries in vitro. Thus, brachial artery dilatation may be considered representative for vascular NO availability.
The influence of resting diameter on FMD is well known Citation[8–10], and probably a larger diameter reflects a lower degree of active tone and therefore diminished ability for dilatation. The present study shows that even small differences in resting diameter may result in large differences in FMD and thus emphasizes the importance of normalizing both FMD and NTG‐induced dilatation to resting baseline diameter. The relation between FMD and flow velocity or shear stress has previously been addressed in healthy individuals Citation[6], Citation[7], Citation[9], Citation[10] as well as in patients with hypertension Citation[4], Citation[5]. The results of some of these studies also imply a non‐linear relation between the applied stimulus and FMD, although not as notable as in the present study. The brachial artery blood flow velocities measured in the present study were similar to those obtained by others Citation[4], Citation[5], Citation[9]. Thus, differences in stimuli cannot explain discrepancies between the studies. The attenuation of FMD at high flow velocities is not due to maximal dilatation of the artery, because NTG is capable of inducing a considerably larger dilatation. Instead, there could be exhaustion of endothelial NO release at high flow velocities or alternatively structural abnormalities may limit dilatation.
It has previously been reported that FMD is reduced in hypertensive patients Citation[20–23]. Overall, our data confirm these findings, but also imply that it may only be true for high flow velocities. Previous studies have also investigated the effect of antihypertensive drugs on FMD using the ultrasound technique Citation[22], Citation[24–27]. Some report improvement in FMD after ACE inhibitor treatment Citation[22], Citation[25], Citation[26], in line with the present study. In contrast, atenolol treatment has not been shown to affect FMD Citation[22], Citation[25], as was the case in the present study. However, factors in addition to shear stress and lowering of BP may affect FMD. ACE inhibitor treatment of essential hypertension has been associated with reduced oxidative stress and possible increased availability of NO Citation[28], whereas beta‐blockers may lack this effect Citation[29], Citation[30]. The difference in NTG‐induced dilatation between patients and controls and the improvement in NTG‐induced dilatation only in perindopril‐treated patients may support this view.
Acetylcholine‐induced vasorelaxation
In small resistance‐sized arteries, like those investigated in the present study, acetylcholine‐induced relaxation is only to a very limited extent dependent on NO, but as we have previously demonstrated in humans, predominantly exerted through non‐NO endothelium‐dependent hyperpolarization Citation[31]. Thus, it could be anticipated that hypertension affects acetylcholine‐induced relaxation differently in the resistance vessels than the flow‐mediated large artery dilatation.
A number of studies have evaluated the effect of hypertension on human small artery endothelial function. Using plethysmography, a reduced vasodilatation response to intra‐arterial acetylcholine has been demonstrated Citation[32], Citation[33], although this could not be confirmed in a larger‐scale study Citation[34]. Also, in vitro studies have suggested a reduced acetylcholine relaxation in small arteries from hypertensive patients Citation[35], Citation[36]. However, as shown in the present study, the effect of hypertension on acetylcholine‐induced relaxation is small, in accordance with recent investigations on rat resistance arteries showing that high pressure almost exclusively affects NO‐mediated vasodilatation without affecting vasodilatation mediated though endothelium‐derived hyperpolarizing factors Citation[37].
The effect of antihypertensive medication on acetylcholine‐induced vasodilatation has previously been investigated in isolated small arteries. Some report small improvements in relaxation using ACE inhibitor treatment Citation[36], Citation[38], whereas others find no effects Citation[15], Citation[39], as was also the case in the present study. However, in contrast to the findings with ACE inhibition, no studies have ever found any effect of beta‐blockade on small artery acetylcholine relaxation Citation[15], Citation[35], Citation[40]. Taken together, the overall effect of both hypertension as well as antihypertensive medication on isolated small artery relaxation to acetylcholine seems either small or absent.
Overall, our data demonstrate a non‐linear relationship between blood flow velocity and FMDcorr in the brachial artery with attenuation of dilatation at higher flow velocities. Furthermore, the present study is the first direct comparison of antihypertensive medication on large and small artery endothelial function in the same population of patients. Our data suggest that when using commonly applied techniques for functional investigation of large and small artery endothelial function, namely FMD and acetylcholine induced vasorelaxation, the effect of antihypertensive drugs is not comparable between the two types of arteries.
Acknowledgments
This work was supported in part by grants from Institut de Recherces Internationales Servier and the Danish Heart Foundation. We acknowledge technical assistance by Bente Mortensen and Jørgen Andresen.
References
- Lewington S., Clarke R., Qizilbash N., Peto R., Collins R. Age‐specific relevance of usual blood pressure to vascular mortality: A meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360: 1903–1913
- Celermajer D. S., Sorensen K. E., Gooch V. M., Spiegelhalter D. J., Miller O. I., Sullivan I. D., et al. Non‐invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 1992; 340: 1111–1115
- Halcox J. P., Schenke W. H., Zalos G., Mincemoyer R., Prasad A., Waclawiw M. A., et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation 2002; 106: 653–658
- Laurent S., Lacolley P., Brunel P., Laloux B., Pannier B., Safar M. Flow‐dependent vasodilation of brachial artery in essential hypertension. Am J Physiol 1990; 258: H1004–H1011
- Mitchell G. F., Parise H., Vita J. A., Larson M. G., Warner E., Keaney J. F, Jr., et al. Local shear stress and brachial artery flow‐mediated dilation: The Framingham Heart Study. Hypertension 2004; 44: 134–139
- Levenson J., Pessana F., Gariepy J., Armentano R., Simon A. Gender differences in wall shear‐mediated brachial artery vasoconstriction and vasodilation. J Am Coll Cardiol 2001; 38: 1668–1674
- Betik A. C., Luckham V. B., Hughson R. L. Flow‐mediated dilation in human brachial artery after different circulatory occlusion conditions. Am J Physiol 2004; 286: H442–H448
- Celermajer D. S., Sorensen K. E., Bull C., Robinson J., Deanfield J. E. Endothelium‐dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol 1994; 24: 1468–1474
- Gnasso A., Carallo C., Irace C., De Franceschi M. S., Mattioli P. L., Motti C., et al. Association between wall shear stress and flow‐mediated vasodilation in healthy men. Atherosclerosis 2001; 156: 171–176
- Pyke K. E., Dwyer E. M., Tschakovsky M. E. Impact of controlling shear rate on flow‐mediated dilation responses in the brachial artery of humans. J Appl Physiol 2004; 97: 499–508
- Buus N. H., Bottcher M., Jorgensen C. G., Christensen K. L., Thygesen K., Nielsen T. T., et al. Myocardial perfusion during long‐term angiotensin‐converting enzyme inhibition or beta‐blockade in patients with essential hypertension. Hypertension 2004; 44: 465–470
- Sorensen K. E., Celermajer D. S., Spiegelhalter D. J., Georgakopoulos D., Robinson J., Thomas O., et al. Non‐invasive measurement of human endothelium dependent arterial responses: Accuracy and reproducibility. Br Heart J 1995; 74: 247–253
- Buus N. H., Bottcher M., Bottker H. E., Sorensen K. E., Nielsen T. T., Mulvany M. J. Reduced vasodilator capacity in syndrome X related to structure and function of resistance arteries. Am J Cardiol 1999; 83: 149–154
- Sorensen K. E., Dorup I., Hermann A. P., Mosekilde L. Combined hormone replacement therapy does not protect women against the age‐related decline in endothelium‐dependent vasomotor function. Circulation 1998; 97: 1234–1238
- Thybo N. K., Stephens N., Cooper A., Aalkjaer C., Heagerty A. M., Mulvany M. J. Effect of antihypertensive treatment on small arteries of patients with previously untreated essential hypertension. Hypertension 1995; 25: 474–481
- Joannides R., Haefeli W. E., Linder L., Richard V., Bakkali E. H., Thuillez C., et al. Nitric oxide is responsible for flow‐dependent dilatation of human peripheral conduit arteries in vivo. Circulation 1995; 91: 1314–1319
- Doshi S. N., Naka K. K., Payne N., Jones C. J., Ashton M., Lewis M. J., et al. Flow‐mediated dilatation following wrist and upper arm occlusion in humans: The contribution of nitric oxide. Clin Sci (Lond) 2001; 101: 629–635
- Thorsgaard M., Lopez V., Buus N. H., Simonsen U. Different modulation by Ca2+‐activated K+ channel blockers and herbimycin of acetylcholine‐ and flow‐evoked vasodilatation in rat mesenteric small arteries. Br J Pharmacol 2003; 138: 1562–1570
- Paniagua O. A., Bryant M. B., Panza J. A. Role of endothelial nitric oxide in shear stress‐induced vasodilation of human microvasculature: Diminished activity in hypertensive and hypercholesterolemic patients. Circulation 2001; 103: 1752–1758
- Muiesan M. L., Salvetti M., Monteduro C., Corbellini C., Guelfi D., Rizzoni D., et al. Flow‐mediated dilatation of the brachial artery and left ventricular geometry in hypertensive patients. J Hypertens 2001; 19: 641–647
- Olsen M. H., Wachtell K., Hermann K. L., Bella J. N., Dige‐Petersen H., Rokkedal J., et al. Left ventricular hypertrophy is associated with reduced vasodilatory capacity in the brachial artery in patients with longstanding hypertension. A LIFE substudy. Blood Press 2002; 11: 285–292
- Ghiadoni L., Magagna A., Versari D., Kardasz I., Huang Y., Taddei S., et al. Different effect of antihypertensive drugs on conduit artery endothelial function. Hypertension 2003; 41: 1281–1286
- Lauer T., Hei C., Preik M., Balzer J., Hafner D., Strauer B. E., et al. Reduction of peripheral flow reserve impairs endothelial function in conduit arteries of patients with essential hypertension. J Hypertens 2005; 23: 563–569
- Muiesan M. L., Salvetti M., Monteduro C., Rizzoni D., Zulli R., Corbellini C., et al. Effect of treatment on flow‐dependent vasodilation of the brachial artery in essential hypertension. Hypertension 1999; 33: 575–580
- Komai N., Ohishi M., Morishita R., Moriguchi A., Kaibe M., Matsumoto K., et al. Serum hepatocyte growth factor concentration is correlated with the forearm vasodilator response in hypertensive patients. Am J Hypertens 2002; 15: 499–506
- Munakata M., Aihara A., Nunokawa T., Ito N., Imai Y., Ito S., et al. The influence of one‐year treatment by angiotensin converting enzyme inhibitor on baroreflex sensitivity and flow‐mediated vasodilation of the brachial artery in essential hypertension – Comparison with calcium channel blockers. Clin Exp Hypertens 2003; 25: 169–181
- Chung N. A., Beevers D. G., Lip G. Effects of losartan versus hydrochlorothiazide on indices of endothelial damage/dysfunction, angiogenesis and tissue factor in essential hypertension. Blood Press 2004; 13: 183–189
- Hornig B., Landmesser U., Kohler C., Ahlersmann D., Spiekermann S., Christoph A., et al. Comparative effect of ace inhibition and angiotensin II type 1 receptor antagonism on bioavailability of nitric oxide in patients with coronary artery disease: Role of superoxide dismutase. Circulation 2001; 103: 799–805
- Taddei S., Virdis A., Ghiadoni L., Magagna A., Pasini A. F., Garbin U., et al. Effect of calcium antagonist or beta blockade treatment on nitric oxide‐dependent vasodilation and oxidative stress in essential hypertensive patients. J Hypertens 2001; 19: 1379–1386
- Baykal Y., Yilmaz M. I., Celik T., Gok F., Rehber H., Akay C., et al. Effects of antihypertensive agents, alpha receptor blockers, beta blockers, angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers and calcium channel blockers, on oxidative stress. J Hypertens 2003; 21: 1207–1211
- Buus N. H., Simonsen U., Pilegaard H. K., Mulvany M. J. Nitric oxide, prostanoid and non‐NO, non‐prostanoid involvement in acetylcholine relaxation of isolated human small arteries. Br J Pharmacol 2000; 129: 184–192
- Panza J. A., Quyyumi A. A., Brush J. E, Jr., Epstein S. E. Abnormal endothelium‐dependent vascular relaxation in patients with essential hypertension. N Engl J Med 1990; 323: 22–27
- Rossi M., Taddei S., Fabbri A., Tintori G., Credidio L., Virdis A., et al. Cutaneous vasodilation to acetylcholine in patients with essential hypertension. J Cardiovasc Pharmacol 1997; 29: 406–411
- Cockcroft J. R., Chowienczyk P. J., Benjamin N., Ritter J. M. Preserved endothelium‐dependent vasodilatation in patients with essential hypertension. N Engl J Med 1994; 330: 1036–1040
- Schiffrin E. L., Deng L. Y. Structure and function of resistance arteries of hypertensive patients treated with a beta‐blocker or a calcium channel antagonist. J Hypertens 1996; 14: 1247–1255
- Rizzoni D., Muiesan M. L., Porteri E., Castellano M., Zulli R., Bettoni G., et al. Effects of long‐term antihypertensive treatment with lisinopril on resistance arteries in hypertensive patients with left ventricular hypertrophy. J Hypertens 1997; 15: 197–204
- Christensen F. H., Hansen T., Stankevicius E., Buus N. H., Simonsen U. Elevated pressure selectively blunts flow‐evoked vasodilatation in rat mesenteric small arteries. Br J Pharmacol 2007; 150: 80–87
- Schiffrin E. L., Deng L. Y., Larochelle P. Effects of a beta‐blocker or a converting enzyme inhibitor on resistance arteries in essential hypertension. Hypertension 1994; 23: 83–91
- Thybo N. K., Mulvany M. J., Jastrup B., Nielsen H., Aalkjaer C. Some pharmacological and elastic characteristics of isolated subcutaneous small arteries from patients with essential hypertension. J Hypertens 1996; 14: 993–998
- Schiffrin E. L., Park J. B., Pu Q. Effect of crossing over hypertensive patients from a beta‐blocker to an angiotensin receptor antagonist on resistance artery structure and on endothelial function. J Hypertens 2002; 20: 71–78