Abstract
Background. ACCOMPLISH is a “new‐generation” hypertension trial assessing single‐tablet combination therapy for initial treatment of high‐risk hypertension. At baseline, 97% of subjects were treated with anti‐hypertensive medication at entry, but only 37% of participants had blood pressure (BP) control (<140/90 mmHg). Single‐tablet combination therapy may improve control rates. Methods. The mean BP change from baseline at the end of 6 months (the time point when subjects should have had all of the drug titrations to achieve BP control) was examined for 10,704 randomized patients. Within‐group changes were examined using t‐tests. Comparisons between subgroups were made using analysis of variance (ANOVA) and covariance (ANCOVA). Results. Mean (±SD) BP fell from 145±18/80±11 mmHg at randomization to 132±16/74±10 mmHg. The 6‐month BP control rate was 73% in the overall trial (78% in the US), 43% in diabetics and 40% in patients with renal disease. Of the patients uncontrolled, 61% were not on maximal medications, suggesting potential increases in control rates. Serious hypotensive events occurred in 1.8% of participants. Conclusion. ACCOMPLISH BP control rates are the highest of any multi‐national trial to date. Whereas current guidelines recommend combination therapy only for stage 2 hypertension, in this trial it is expedient and safe for both stage 1 and 2 hypertension.
Introduction
The ACCOMPLISH trial has been designed to compare the efficacy of two types of antihypertensive drug combinations in preventing major clinical outcomes in hypertensive patients at high risk of cardiovascular events. The two formulations being compared are comprised of the calcium‐channel blocker amlodipine besylate, combined with the angiotensin‐converting enzyme (ACE) inhibitor benazapril; and benazapril combined with the diuretic hydrochlorothiazide. The hypothesis for this study is that the calcium‐channel blocker/ACE inhibitor combination will be more effective than the diuretic combination in reducing major cardiovascular outcomes in part, by improving vascular function through a synergistic effect of both amlodipine and benazapril on nitric oxide availability Citation[1]. This trial design contrasts current diuretic‐based treatment strategies, which assume that the addition of an ACE inhibitor to a diuretic will abrogate the untoward metabolic effects of diuretics. The detailed rationale supporting the ACCOMPLISH hypothesis has been published previously Citation[2].
The recruitment for ACCOMPLISH has been completed and the characteristics of the patients entering this trial have been described Citation[3]. This cohort is unique in that the subjects were recruited after several clinical trials proved that cardiovascular risk could be significantly attenuated by specific drugs. Accordingly, ACCOMPLISH participants had high utilization of ACE inhibitors/angiotensin II receptor blockers (78%), statins (67%) and anti‐platelet therapy (63%) at entry into the trial. The mean low‐density lipoprotein (LDL)‐cholesterol value was 102 mg/dl, whereas high‐density lipoprotein (HDL) was 50 mg/dl. Virtually all (97% of subjects) were treated for hypertension (74% on two or more drugs) prior to trial entry Citation[3]. The baseline features of the cohort converge to suggest that clinicians are adopting aggressive treatment strategies. However, despite the attempt at aggressive treatment, the overall baseline blood pressure (BP) control rate was only 37%. Taken together with the observation that ACCOMPLISH subjects are obese (average body mass index, BMI = 31) and 60% were diabetic, it is possible that secular trends in obesity and diabetes (DM) may be creating a population, more resistant to the traditional strategies for BP control.
The ACCOMPLISH trial is “new generation” in its design by randomizing subjects to single‐pill dual therapy. The traditional approach to hypertension management has been to initiate monotherapy then sequentially use additional medications as needed in order to achieve a target BP goal (JNC7) Citation[4]. While previous hypertension trials have had some success in attenuating cross‐contamination of secondary and tertiary drugs between trial arms, such contamination often leads to BP differences in study arms and makes interpretation of the results quite difficult. Parenthetically, treatment with initial combination therapy has been reported to result in prompt and robust reductions in BP Citation[5]. The efficacy of initial combination therapy in the obese, mostly diabetic subjects, with both stage 1 and stage 2 hypertension in the ACCOMPLISH trial, is the subject of the present report.
Methods
A full description of the ACCOMPLISH methods has been published previously Citation[2]. The following is a brief description. ACCOMPLISH is a randomized, double‐blind trial that will compare the efficacy of the amlodipine besylate/benazapril combination with that of a benazapril/hydrochlorothiazide combination in preventing fatal and non‐fatal cardiovascular outcomes. Patients treated for hypertension were switched to randomized study medication without a washout period. Patients eligible to enter the study have their drug doses force‐titrated during the first 2 months of the trial to maximum ACE inhibitor levels: amlodipine besylate/benazapril 5/40 mg, and benazapril/hydrochlorothiazide 40/12.5 mg. Within the first 3 months, the doses could be increased to 10/40 mg or 40/25 mg, respectively, and other antihypertensive agents (excluding the drug classes involved in the primary treatments, but including beta‐blockers, alpha‐blockers, clonidine and, if needed, loop diuretics) could then be added in order to reach BP targets (<140/90 mmHg for most patients, <130/80 mmHg [suggested, but not mandated] for patients with DM or renal insufficiency). After the initial 3‐month period, patients were seen again at 6 months after the start of study and thereafter at 6‐month intervals until the end of the 5‐year trial.
The data in this report are based on all available blood pressure observations at baseline (the beginning of the study treatment period immediately following randomization) and at 6 months. A total of 10,704 subjects had BP data available for the present analysis. The primary and other mortality/morbidity endpoints of this study are not discussed in this report.
The total number of patients randomized in the trial (a total of approximately 11,500 patients randomized to two treatment groups) reflects power calculations based on testing the principal study hypothesis for the primary cardiovascular mortality/morbidity endpoint; the details of this calculation have been published Citation[2]. The sample size is many times larger than the sample size that would be required to detect between‐group differences in variables, such as systolic blood pressure (SBP).
Patient selection
The inclusion and exclusion criteria for hypertensive patients at high cardiovascular risk have been described previously Citation[2]. In general, ACCOMPLISH participants are hypertensive, age >60 years, and have cardiovascular or renal disease, or two target organs damaged by hypertension.
Statistical considerations
The statistical analyses described below are exploratory analyses performed on blinded, pooled‐treatment data. Changes in mean SBP from baseline to 6 months were compared for all randomized patients and for selected patient subgroups. Tests of the null hypothesis of zero mean change in SBP within each group are based on Student's t‐test. Tests comparing the difference in mean SBP change from baseline between selected patient subgroups are based on F‐tests from an analysis of covariance (ANCOVA), adjusting for patients' baseline SBP values. Subgroup comparisons of mean absolute SBP at baseline, based on F‐tests from an analysis of variance (ANOVA), were also calculated.
BP control rates (for SBP/DBP <140/90 mmHg) were examined at baseline and 6 months. BP control was defined as meeting the criteria of both SBP<140 mmHg and diastolic BP (DBP) <90 mmHg. Separate analyses were also performed for the SBP/DBP<130/80 mmHg control rate for subjects with DM and chronic kidney disease (CKD).
Within‐group comparisons of baseline versus 6‐month control status are based on chi‐square tests. Between‐subgroup comparisons for baseline “yes/no” control status are based on Mantel–Haenszel chi‐square tests for group mean differences (for integer‐valued categories). Similar between‐group comparisons for 6‐month control status are given, based on Cochran–Mantel–Haenszel chi‐square tests for group mean differences controlling for baseline BP control status.
Results
Effects of initial combination therapy on blood pressure
The effects on SBP after 6 months of treatment are shown in Table . The data are shown for the entire cohort as well as for subgroups of interest. The baseline values were measured before initiation of study drugs while patients were still receiving pre‐study medications. The baseline BP values were similar across most subgroups, with the exception of the subjects enrolled at the Nordic sites, where SBPs at the time of randomization were approximately 10 mmHg higher than in patients from the USA. The higher baseline BP levels in the Nordic countries were due to local regulatory advice to include only subjects in the trial that were not controlled on their pre‐study medications, while in the USA, investigators could enroll both well controlled or uncontrolled subjects.
Table I. Effects of initial combination therapy on systolic blood pressure at 6 months in ACCOMPLISH.
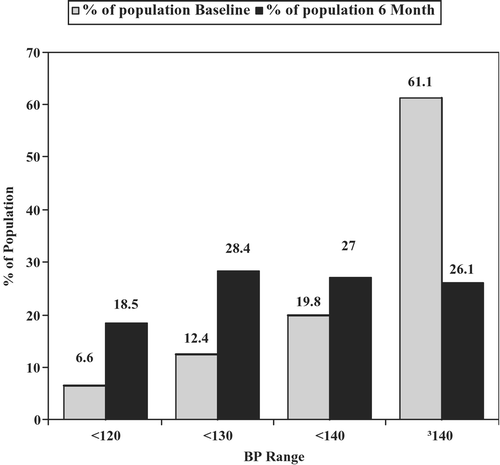
For the total study population, as well as for each of the subgroups listed in Table , mean SBP at 6 months was significantly reduced compared with baseline (p<0.001 for all groups): from 145.5/80.2 to 132.5/74.3 mmHg (p<0.001). The magnitude of absolute BP reduction was greater in the Nordic countries when compared with the US patients. This may reflect the significantly higher mean SBP baseline values for the Nordic countries (F = 776.54, p<0.0001). Neither age nor gender appeared to influence the effects on SBP. Similar trends were noted for diastolic BP. Presumably reflecting the forced titration from the initial to the intermediate combination doses, 3262 patients (18.5%) finished with 6‐month SBPs below 120 mmHg, and 5599 (46.9%) below 130 mmHg, compared with baseline values, where 60% of subjects had SBP ⩾140 mmHg (Figure ).
Effects of initial combination therapy on blood pressure control rates
The achieved BP control rates are shown in Table . Overall, 73% of patients achieved blood pressure control at 6 months. In the US cohort, control rates exceeded 78%. The Nordic patients, despite greater reductions in BP during treatment, had a lower control rate (62%) than patients from the USA (chi‐square = 149.5, p<0.0001) because of their lower control rate at baseline (21% Nordic vs. 44% US; chi‐square = 534.4, p<0.0001). Neither age nor gender appeared to markedly affect blood pressure control, and likewise the outcomes in black and white patients were similar. While there are only few Asian participants, 91% of them achieved BP control.
Table II. Effects of initial combination therapy on blood pressure control rates at 6 months in ACCOMPLISH.
Drug usage during titration
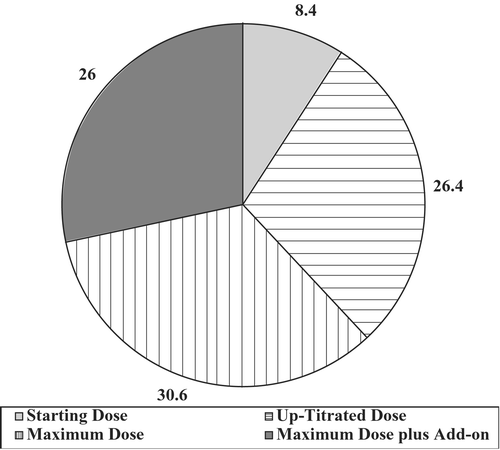
The actual usage of drugs during the 6‐month titration period is shown in Figure . By 6 months, 2850 patients (26%) were receiving add‐on treatment. Most of these patients (2223) were receiving just one drug; 291 were receiving two, 213 were receiving three, and 123 were receiving four or more add‐on drugs. With respect to the add‐on therapy, investigators were given the discretion to select from beta‐blockers, alpha‐blockers, and clonidine and loop diuretics. The beta‐blockers were the most commonly selected agents, although there was also wide usage of alpha‐blockers and loop diuretics; clonidine was used less frequently than the other agents.
Despite the broad use of the maximum combination doses, as well as the add‐on drugs, at 6 months 1743 (61%) of the 2869 patients not yet achieving goal BPs had not been offered treatment with the maximum combination dose or add‐on therapy. Indeed, 717 of these patients (25.0% of non‐controlled) had not yet been titrated to the maximum dose of study medication.
At randomization (prior to month 1), 84 patients (0.7%) discontinued their treatment; and by 1, 2, 3 and 6 months, the cumulative discontinuations had grown, respectively, to 184 (1.7%), 244 (2.1%), 345 (3.0%) and 457 (4.0%).
Safety
The most commonly reported side‐effects are presented in Table . Only 1.8% of subject reported symptoms consistent with hypotension. Cough, edema and dizziness were reported with equal frequency.
Table III. Most frequent adverse events at 6 months in ACCOMPLISH (>1%).
Discussion
The treatment regimens used in this trial have reduced the mean blood pressure in a large cohort of high‐risk hypertensive patients to 132/74 mmHg by 6 months. Importantly, these subjects were on average obese, diabetic and on multiple antihypertensive medications at enrollment into the trial. A key factor in achieving this result is the use of two‐drug antihypertensive combinations to initiate therapy immediately following patient randomization. The mean BP immediately prior to the start of study treatment was already at a relatively low level of 145/80 mmHg, indicative of the fact that almost all patients entering this trial were already receiving active treatment, most often with multiple drugs, for their hypertension Citation[3]. Starting study treatment with combination therapy appears to be a more effective strategy for BP management than starting with single agents Citation[6–8].
A further contributor to the high BP response rates observed in this trial was the forced titration of the combination drug regimens from an initial to an intermediate dose level. Patients randomized to the amlodipine/benazepril treatment were required to have their doses increased from 5/20 mg to 5/40 mg after 1 month; and those starting with benazepril/hydrochlorothiazide, from 20/12.l5 mg to 40/12.5 mg. The rationale for this forced titration was primarily to ensure that patients received a similar dose of ACE inhibitor without regard for level of achieved BP Citation[2]. The forced titration had an additional effect: for those patients whose BPs were not yet controlled by initial therapy, this up‐titration provided additional antihypertensive efficacy, and for those patients whose BPs were controlled by the initial therapy, even further reductions were produced. In fact, by the end of 6 months of treatment, 18.5% of patients had SBP values below 120 mmHg, and 47% were below 130 mmHg. The overall rate of hypotension was 1.8%. These findings indicate that for a meaningful proportion of high‐risk hypertensive patients, it is possible to achieve BP values substantially below the levels currently recommended as targets by published hypertension guidelines without undue risk of hypotension Citation[4], Citation[9].
The goal BP of <140/90 mmHg was achieved within 6 months in 73% of the patients in this study. Of note, this target was achieved similarly in men and women, patients aged below or above 70, and in white and black patients. In the US cohort, control rates improved from 44% (consistent with NHANES) Citation[10] to greater than 78% (unprecedented in clinical trials). Previous BP studies had indicated that treatment with antihypertensive drug combinations with complementary actions, including blockers of the renin–angiotensin system paired with either diuretics or calcium‐channel blockers, would work equally well across most demographic hypertensive groups Citation[11], Citation[12].
Emphasis should be placed on the blood pressure results in diabetic patients, since they comprise about 60% of the study cohort. When judged by the criterion of <140/90 mmHg, these patients had a 72% control rate by 6 months, similar to the overall cohort. In keeping with contemporary hypertension guidelines, the protocol for this study had recommended that BPs in diabetic patients be reduced to a target of <130/80 mmHg Citation[4], Citation[9]. This goal, however, was not a mandatory requirement of the protocol, and so it is not entirely surprising that the control rate in diabetic patients judged by this stringent BP level was 43%. It is noteworthy that the diabetic cohort achieved a SBP of 132.8 mmHg on average suggesting that control to the guideline target of <130 mmHg is attainable for most diabetics. Continuing efforts by the investigators in this trial should almost certainly increase the percentage of responders among diabetic (and non‐diabetic) patients as the study progresses.
In considering control rates, it is relevant to ascertain if all available therapeutic options have been exercised to bring each patient's BP below the desired level. In fact, 61% of the non‐controlled participants had still not been offered maximum therapy, and 25% of these patients had not even been titrated to the top dose of their initial study medication. It must be recognized, however, that investigators bear the responsibility for the overall well‐being of their patients. In some cases they might have made judgments based on factors such as treatment side‐effects or concomitant medical conditions to withhold or delay the use of more aggressive therapies. It is likely, as the trial progresses, that a growing proportion of the non‐controlled patients will have their treatment regimens advanced. This has happened in other trials Citation[5], Citation[8], Citation[13], Citation[14]. Aggressive BP control campaigns have been launched in the ACCOMPLISH trial to improve BP control rates over the course of the trial.
It is difficult to directly compare blood pressure results among major clinical outcomes studies in high‐risk hypertensive patients, but ACCOMPLISH has achieved higher BP control rates than any other large study of hypertensive participants (Table ) Citation[15–23]. These large BP trials have used differing study designs, patient entry criteria and treatment algorithms, so that it may not be appropriate to compare directly BP outcomes, particularly after only 6 months of treatment.
Table IV. Achieved blood pressure in recent multi‐center clinical trials.
Overall, this report of BP effects during the first 6 months of treatment in the ACCOMPLISH trial indicates that the algorithm of initial combination therapy, either benazepril with amlodipine, or with hydrochlorothiazide, is rapidly effective and safe in producing BP control in a majority of hypertensive patients. In general, the achieved BP levels in this study exceed previous major trials in hypertension reported during recent years. Quite apart from the issue of the relative merits of the two treatment arms in preventing major clinical endpoints, the experience of this trial so far indicates that initiating antihypertensive therapy in high‐risk patients with fixed combinations is a highly effective strategy. It is likely that these findings will strengthen the recommendations published in national guidelines to consider the use of drug combinations for initial treatment Citation[4], Citation[9]. Moreover, our data suggest expanding the usage of initial combination therapy to high‐risk subjects with stage 1 hypertension.
Acknowledgements
We thank all trial participants, physicians, nurses and practices in the participating countries. The study was supported by the principal funding source, Novartis Pharma AG. The ACCOMPLISH Trial is investigator‐initiated. The protocol was developed by the Executive Committee and registered with the FDA. The original draft and all revisions of this manuscript were done by the Executive Committee. Novartis medical officers and clinical trial scientists are non‐voting attendees at Duke Clinical Research Institute. Study endpoints are adjudicated by an independent committee at Brigham and Women's Hospital. We thank Roxanne Kelly, Linda Staikos and Elizabeth Williamson for their assistance in typing and collating the report.
References
- Zhang X., Xu X., Nasjletti A., Hintze T. H. Amlodipine enhances NO production induced by an ACE inhibitor through a kinin‐mediated mechanism in canine coronary microvessels. J Cardiovasc Pharm 2000; 35: 195–202
- Jamerson K. A., Bakris G. L., Wun C. C., Dahlof B., Lefkowitz M., Manfreda S., et al. Rationale and design of the Avoiding Cardiovascular events through COMbination therapy in Patients LIving with Systolic Hypertension (ACCOMPLISH) trial. Am J Hypertens 2004; 17: 793–801
- Weber M. A., Bakris G. L., Dahlöf B., Pitt B., Velazquez E., Gupte J., et al. Baseline characteristics in the Avoiding Cardiovascular events through COMbination therapy in Patients LIving with Systolic Hypertension (ACCOMPLISH) trial: A hypertensive population at high cardiovascular risk. 2006 (in press), Blood Pressure
- Chobanian A. V., Bakris G. L., Black H. R., Cushman C., Green L. A., Izzo J. L., et al. and the National High Blood Pressure Education Program Coordinating Committee. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. The JNC7 Report. JAMA 2003; 289: 2560–2572
- Jamerson K. A., Nwose O., Jean‐Louis L., Schofield L., Purkayastha D., Baron M. Initial angiotensin‐converting enzyme inhibitor/calcium channel blocker combination therapy achieves superior blood pressure control compared with calcium channel blocker monotherapy in patients with stage 2 hypertension. Am J Hypertens 2004; 17: 495–501
- The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs. diuretic: The Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002; 288: 2981–2997
- Weber M. A. The ALLHAT Report: A case of information and misinformation. J Clin Hypertens 2003; 5: 9–13
- Julius S., Kjeldsen S., Weber M., Brunner H. R., Ekman S., Hansson L., et al. for the VALUE trial group. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: The VALUE randomized trial. Lancet 2004; 363: 2022–2031
- 2003 European Society of Hypertension–European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003; 21: 1011–1054
- Burt V. L., Whelton P., Roccella E. J., Brown C., Cutler J. A., Higgins M., et al. Prevalence of hypertension in the US adult population; Results from the Third National Health and Nutrition Examination Survey. Hypertension 1995; 25: 305–313
- Neutel J. M., Smith D. H. G., Weber M. A. Effect of antihypertensive monotherapy and combination therapy on arterial distensibility and left ventricular mass. Am J Hypertens 2004; 17: 37–42
- White W. B., Giles T. D., Bakris G. L., Neutel J. M., Davidai G., Weber M. A. Measuring the efficacy of antihypertensive therapy by ambulatory blood pressure monitoring in the primary care setting. Am Heart J 2006; 151: 176–184
- Dahlöf B., Sever P. S., Poulter N. R., Wedel H., Beevers D. G., Caulfield M., et al. for the ASCOT Investigators. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo‐Scandinavian Cardiac Outcomes Trial‐Blood Pressure Lowering Arm (ASCOT‐BPLA): A multicentre randomized controlled trial. Lancet 2005; 366: 895–906
- Poulter N. R., Wedel H., Dahlöf B., Sever P. S., Beevers D. G., Caulfield M., et al. for the ASCOT Investigators. Role of blood pressure and other variables in the differential cardiovascular event rates noted in the Anglo‐Scandinavian Cardiac Outcomes Trial‐Blood Pressure Lowering Arm (ASCOT‐BPLA). Lancet 2005; 366: 907–913
- Hansson L., Zanchetti A., Carruthers S. G., Dahlof B., Elmfeldt D., Julius S., et al. for the HOT Study Group. Effects of intensive blood‐pressure lowering and low‐dose aspirin in patients with hypertension: Principal results of the Hypertension Optimal Treatment (HOT) randomized trial. Lancet 1998; 351: 1755–1762
- Hansson L., Lindholm L. H., Niskanen L., Lanke J., Hedner T., Nikalson A., et al. for the Captopril Prevention Project (CAPPP) study group. Effect of angiotensin‐converting enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: The Captopril Prevention Project (CAPPP) randomized trial. Lancet 1999; 353: 611–616
- Hansson L., Lindholm L. H., Ekbom T., Dahlof B., Lanke J., Schersten B., et al. for the STOP‐Hypertension‐2 study group. Randomized trial of old and new antihypertensive drugs in elderly patients: Cardiovascular mortality and morbidity, the Swedish Trial in Old Patients with Hypertension‐2 study. Lancet 1999; 354: 1751–1756
- Hansson L., Hedner T. M., Lund‐Johansen P., Kjeldsen S. E., Lindholm L. H., Syvertsen J. O., et al. for the NORDIL Study Group. Randomized trial effects of calcium antagonists compared with diuretics and (‐blockers on cardiovascular morbidity and mortality in hypertension: The Nordic Diltiazem (NORDIL) study. Lancet 2000; 356: 359–365
- Brown M. J., Palmer C. R., Castaigne A., de Leeuw P. W., Mancia G., Rosenthal T., et al. Morbidity and mortality in patients randomized to double‐blind treatment with a long‐acting calcium‐channel blocker or diuretic in the International Nifedipine GITS study: Intervention as a Goal in Hypertension Treatment (INSIGHT). Lancet 2000; 356: 366–372
- Dahlöf B., Devereux R. B., Kjeldsen S. E., Julius S., Beevers G., de Faire U., et al. for the LIFE study group. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): A randomized trial against atenolol. Lancet 2002; 359: 995–1003
- Cushman W. C., Ford C. E., Cutler J. A., Margolis K. L., Davis B. R., Grimm R. H., et al. for the ALLHAT Research Group. Success and predictors of blood pressure control in diverse North American settings: The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). J Clin Hypertens 2002; 4: 393–404
- Weber M. A., Julius S., Kjeldsen S. E., Brunner H. R., Ekman S., Hansson L., et al. Blood pressure dependent and independent effects of antihypertensive treatment on clinical events in the VALUE trial. Lancet 2004; 363: 2049–2051
- Sever P. S., Dahlöf B., Poulter N. R., Wedel H., Beevers G., Caulfield M., et al. for the ASCOT Investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower‐than‐average cholesterol concentrations, in the Angio‐Scandinavian Cardiac Outcomes Trial–Lipid lowering Arm (ASCOT‐LLA): A multicentre randomized controlled trial. Lancet 2003; 361: 1149–1158