Abstract
Background. Hyperuricemia is associated with hypertension, vascular disease and cardiovascular (CV) disease. However, the role of serum uric acid (SUA) level as an independent risk factor for CV and renal morbidity in hypertension remains controversial. Accordingly, we aimed to determine whether SUA levels are independently and specifically associated with coronary flow reserve (CFR) impairment in hypertensive patients. Methods. We examined 80 never treated and newly diagnosed hypertensive individuals. The hypertensive individuals were divided into two groups based on CFR values. Results. Subjects with altered CFR (<2) had significantly higher SUA levels compared with those with normal CFR (⩾2) (346.0±98.1 vs 260.7±75.6 µmol/l, p<0.0001). After adjusting for potential confounders, including age, sex, body mass index, blood pressure, lipids and creatinine, we found that SUA levels were independently associated with CFR impairment (β = −0.417, p<0.0001). We also found that SUA levels were a good predictor of low CFR at the receiver‐operating characteristic curve. Area under the curve was 76% (95% CI 0.64–0.88), and SUA levels were significantly predictive of low CFR (p<0.0001). Conclusions. These results support a role for SUA level as an independent marker of target organ damage in hypertension.
Introduction
Substantial evidence suggests a significant relationship between increased serum uric acid (SUA) levels and cardiovascular (CV) morbidity and mortality. During the last 50 years, clinical and epidemiological studies have convincingly showed a positive association of SUA levels with myocardial infarction, stroke and all CV events in the general population, particularly in patients with hypertension, diabetes mellitus and heart failure Citation[1–10]. In addition, elevated SUA levels are commonly associated with hypertension. Twenty‐five percent of untreated hypertensive individuals have elevated SUA levels Citation[11].
It has been suggested that impairment of coronary flow reserve (CFR) may occur very early in hypertension before hypertrophy is apparent, and therefore may cause subsequent ischemia and fibrosis Citation[12]. Reduced CFR is largely the result of changes in minimal coronary resistance that are independent of vascular tone Citation[12–15]. Therefore, structural changes in the coronary vasculature are most likely to be the major contributors to impaired CFR. These structural changes may be qualitatively similar to the well‐described effects of hypertension on the peripheral circulation Citation[16]. Furthermore, quantitative histological studies performed on septal biopsy tissue showed that reduced coronary dilatory capacity was associated with increased arteriolar media area, and perivascular and interstitial fibrosis in patients with arterial hypertension and angina pectoris in the absence of relevant coronary artery stenosis Citation[13]. These conditions are sensitive indicators of hypertensive end‐organ damage Citation[13], Citation[14].
The presence of subclinical hypertensive target organ damage signals a condition of increased risk for CV and renal morbidity and mortality Citation[17]. Therefore, the search for impaired CFR may be recommended as part of global risk assessment. Since the role of SUA in the development of CV disease is still receiving growing attention, a better understanding of its relationship with hypertensive target organ damage may help to clarify the pathophysiological mechanisms underlying this association. Thus, the present study was performed to evaluate the association between SUA levels and impaired CFR in a group of middle‐aged never treated and newly diagnosed patients with essential hypertension.
Methods
Study population
The overall study population was consisted of 80 never treated and newly diagnosed essential hypertensive subjects. In each subject, blood pressure (BP) was measured on three separate days in a week after 15 min of comfortably sitting and averaged. Individuals who had diastolic BP (DBP) ⩾90 mmHg and/or systolic BP (SBP) ⩾140 mmHg in the office setting were diagnosed as hypertensive. A complete physical examination was performed, with particular attention to peripheral arterial pulses and carotid bruits. Each subject was questioned about major CV risk factors including family history of coronary artery disease (CAD), current smoking status, alcohol consumption and diabetes mellitus. Family history of CAD was obtained by questioning the subjects about CAD in first‐grade male relatives before 55 years and in female relatives before 65 years of age. Age, gender, and BMI were recorded. Fasting blood glucose, total cholesterol, high‐density lipoprotein (HDL)‐cholesterol, low‐density lipoprotein (LDL)‐cholesterol and triglyceride levels were recorded. Plasma levels of C‐reactive protein were measured by use of a highly sensitive sandwich ELISA technique Citation[18]. Blood samples were drawn by venipuncture and centrifuged at 4°C. SUA levels were determined using the usual validated enzymatic colorimetric method on an Aeroset Clinical Chemistry Analyzer (Abbott Lab., Abbott Park, IL, USA).
Inclusion criteria included 18–55 years of age, and regular menstrual cycle for women. Exclusion criteria included presence of any systemic disease such as hemolytic, hepatic and renal diseases or any disease that could impair CFR (e.g. diabetes mellitus: fasting plasma glucose level measured on three separate days in a week >126 mg/dl (7.0 mmol/l) or impaired oral glucose tolerance test: fasting plasma glucose <126 mg/dl (7.0 mmol/l) but 2‐h plasma glucose after a 75‐g oral glucose challenge >140 mg/dl (7.8 mmol/l), family history of CAD and excessive alcohol consumption (>120 g/day). Subjects were excluded from the study if they used any vasoactive drug, had undergone previous anti‐hypertensive therapy, were current smokers, and had ST segment or T wave changes specific for myocardial ischemia, Q waves, and incidental left bundle branch block on ECG. Individuals were also excluded if they had HDL‐cholesterol levels <0.78 mmol/l (30 mg/dl), LDL‐cholesterol levels >4.16 mmol/l (160 mg/dl), triglyceride levels >4.56 mmol/l (400 mg/dl), elevated liver enzymes, body mass index (BMI) greater than 30 kg/m2, or left ventricular (LV) mass index (LVMI) ⩾125 g/m2 for men and 110 g/m2 for women. Written informed consent was obtained from each subject. The institutional ethics committee approved the study protocol.
Echocardiographic examination
Each subject was examined using an Acuson Sequoia C256® Echocardiography System equipped with 3V2c and 5V2c broadband transducers with second harmonic capability (Acuson Corp, Mountain View, CA, USA). Two‐dimensional, M‐mode, and subsequent transthoracic Doppler echocardiographic examinations were performed on each subject.
LV mass determination
LV mass (LVM) was calculated from M‐mode records taken on parasternal long‐axis images according to the formula below Citation[19], and LVMI was expressed as LVM per square meter of body surface area:
CFR measurement
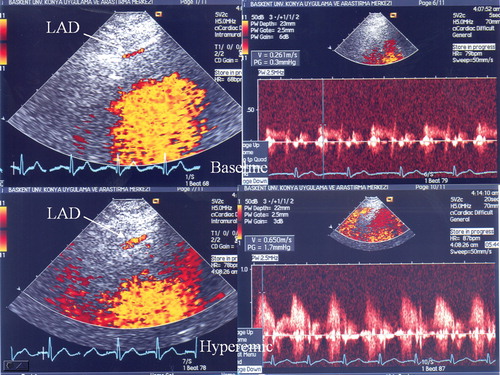
Visualization of the distal left anterior descending (LAD) coronary artery was performed using a modified, foreshortened, two‐chamber view obtained by sliding the transducer on the upper part and medially from an apical two‐chamber view to reach the best alignment to the interventricular sulcus. Subsequently, coronary flow in the distal LAD was examined by color Doppler flow mapping over the epicardial part of the anterior wall, with the color Doppler velocity range 8.9–24.0 cm/s (Figure ). The color gain was adjusted to provide optimal images. The acoustic window was placed at approximately the midclavicular line, in the fourth and fifth intercostal spaces, with the subject in the left lateral decubitus position Citation[20]. The left ventricle was imaged on the long‐axis cross‐section, and the ultrasound beam was then inclined laterally. Next, coronary blood flow in the LAD (middle to distal) was searched by color Doppler flow mapping. All subjects had Doppler recordings of the LAD with a dipyridamole infusion at a rate of 0.56 mg/kg over 4 min. All subjects had continuous heart rate and electrocardiographic monitoring as well as BP recordings at baseline, during dipyridamole infusion, and at recovery. Echocardiographic images were recorded on VHS videotapes. Two experienced echocardiographers, who had been blinded to the clinical data, analyzed the recordings. Placing the sample volume on the color signal allowed spectral Doppler waveforms of the LAD to reveal the characteristic biphasic flow pattern with larger diastolic and smaller systolic components. Coronary diastolic peak velocities were measured at baseline and after dipyridamole (0.56 mg/kg over 4 min) by averaging the highest three Doppler signals for each measurement. CFR was defined as the ratio of hyperemic to baseline diastolic peak velocities (Figure ). CFR ⩾2.0 was considered normal Citation[20–22]. The hypertensive subjects were divided into two groups based on CFR values: 28 subjects with impaired CFR (<2) (group I), and 52 subjects with normal CFR (⩾2) (group II). CFR measurement was achieved in all subjects (100%). To test the coefficient of repeatability of the CFR measurement, in 10 subjects the measurement was repeated two days later. Intra observer intra‐class correlation coefficient for coronary flow measurement was 0.884, and for CFR value it was 0.899.
Statistical analyses
All analyses were conducted using SPSS 9.0 (SPSS for Windows 9.0, Chicago, IL). All group data are expressed as mean±standard deviation. The two groups were compared using the Student t‐test for multiple comparisons. Chi‐square statistics were used to assess differences between categorical variables. Pearson's correlation analysis was used to test univariate relations. Prediction of variables was obtained by stepwise, forward, multiple regression including potential confounders. The receiver‐operating characteristic (ROC) curve was determined to evaluate the predictive performance of SUA to detect low CFR. The area under the ROC curve (AUC), and its standard error were calculated. A p‐value of <0.05 was considered significant.
Results
Clinical characteristics of the study population
The general characteristics and risk factors for CAD of the groups are presented in Table . The following were similar within the impaired and normal CFR groups: age, gender, BMI, SBP, DBP, heart rate, lipid profiles, hemoglobin and fasting levels of glucose. Serum high‐sensitivity C‐reactive protein (hsCRP) level was slightly higher in the impaired CFR group than in the normal CFR group (Table ).
Table I. Demographic and biochemical characteristics of the two groups, showing similarities.
Analyses of echocardiographic measurements
Interventricular septum thickness, left ventricle posterior wall thickness, and LVMI were slightly greater in the impaired CFR group than in the normal CFR group. LV end diastolic diameter, LV systolic diameter, left atrium diameter, and LV ejection fraction were similar between the impaired and normal CFR groups. Mitral E max was slightly different, and mitral E/A ratio and mitral E deceleration time were significantly different between the groups (Table ).
Table II. Data from echocardiographic examinations of the study subjects.
Analysis of CFR measurements
Baseline and peak heart rate and BPs were similar between the two groups. Baseline diastolic peak flow velocity (DPFV) of LAD was similar between the impaired CFR and normal CFR groups. However, hyperemic DPFV and CFR were significantly higher in the normal CFR group than in the impaired CFR group (Table ).
Relationship between SUA levels and CFR
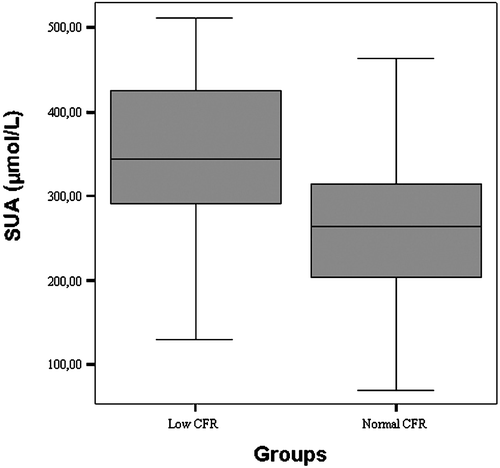
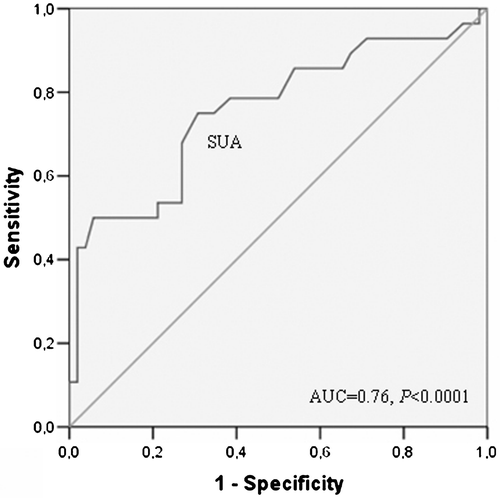
SUA levels were significantly higher in the impaired CFR group than in the normal CFR group (346.0±98.1 vs 260.7±75.6 µmol/l, p<0.0001) (Figure ). In addition, SUA levels were inversely and significantly correlated with CFR (r = −0.422, p<0.0001). Furthermore, in stepwise linear regression analysis, when CFR was taken as dependent, and SUA and other study variables including age, sex, SBP, DBP, LVMI, heart rate, creatinine, hsCRP and lipids (total cholesterol, HDL‐cholesterol, LDL‐cholesterol and triglyceride) as independent, we found that only SUA level was independently correlated with CFR (β = −0.417, p<0.0001). We also demonstrated that SUA levels were a good predictor of low CFR at the ROC curve. Area under the curve (AUC) was 76% (95% confidence interval, CI 0.64–0.88), and SUA levels were significantly predictive of low CFR (p<0.0001) (Figure ).
Discussion
The present study used second harmonic transthoracic Doppler echocardiography (TTDE) for CFR determination to evaluate the possible association between SUA levels and CFR in arterial hypertension where changes in afterload, renal and LV structure may influence coronary blood flow supply. Hypertensive subjects were divided into two groups based on CFR values, with the cut‐off point or normal CFR ⩾2 Citation[20–22].
The main findings of the study are that (i) hypertensive subjects with impaired CFR have elevated SUA levels, and (ii) an independent association between SUA levels and CFR is evident in essential hypertensive individuals, who were newly diagnosed, had never taken any antihypertensive therapy including diuretics and did not have any systemic disease except hypertension. To our knowledge, this is the first study showing an independent association between SUA levels and CFR. Accordingly, SUA levels were significantly different in our hypertensive subjects with normal and altered CFR.
Fifty years ago, it was acknowledged that patients with CAD had increased SUA levels. In addition, elevated SUA levels were discovered to be associated with hypertension, renal disease and obesity, which are frequently seen in patients with CAD. Accordingly, it has since been shown that the patients with elevated SUA levels had a mean 10‐fold increased risk of developing CAD or hypertension, and that gout incidence was threefold higher in hypertensive individuals compared with normotensive subjects Citation[23], Citation[24]. However, evidence is contradictory regarding whether SUA level is an independent risk factor for the development of CAD and hypertension because in two epidemiological studies, hyperuricemia could not be recognized as an independent CV risk factor. A recent analysis from the NHANES III study ensured relevant knowledge to clarify whether SUA level is an independent risk factor for the development of CAD and hypertension. This analysis revealed that hypertensive individuals with SUA levels between 5.0 and 6.9 mg/dl and >7.0 mg/dl had a significantly higher relative risk for both heart attack and stroke (1.32–1.15 and 1.5–2.2, respectively) Citation[25]. These results strongly support the hypothesis that elevated SUA level is an independent risk factor for hypertension associated mortality and morbidity. Confirming these results, we found an independent significant inverse correlation between SUA levels and CFR impairment, which is a surrogate marker of target organ damage in hypertension.
The mechanisms underlying the increase in SUA levels and its potential prognostic implications in essential hypertensive subjects are still not completely known. A direct association exists between SUA and renal hemodynamics in subjects with essential hypertension Citation[26]. Elevated SUA levels in asymptomatic and uncomplicated subjects with essential hypertension may reflect early renal vascular alterations and disturbed renal hemodynamics, with reduction in cortical blood flow and depressed tubular secretion of urate caused by its reduced delivery to the tubular secretory sites Citation[27]. Only hyperuricemic, but not normouricemic hypertensive subjects have showed increased renal vascular resistance. Furthermore, hyperuricemia is related directly to renal vascular resistance and inversely with renal blood flow Citation[26]. In addition, subjects with elevated SUA levels have the most severe renal and systemic vascular involvement Citation[25]. Moreover, Mattei and coworkers reported a significant direct relationship between SUA levels and microalbuminuria, which indicates vascular damage in essential hypertension Citation[28]. Messerli and coworkers Citation[26] hypothesized that the frequent presence of increased SUA levels in hypertensive individuals reflects underlying renal dysfunction or reduced renal perfusion. Thus, it is possible that uric acid may be an earlier and more sensitive marker of decreased renal blood flow than serum creatinine levels, and that elevated SUA levels in hypertension most likely indicate target organ damage and renal vascular involvement. In addition, it is possibility that uric acid may also have a causal role in this process, based on recent data that UA impairs endothelial function by inhibiting the production of nitric oxide (NO) and activating of circulatory platelets Citation[11], Citation[29]. Furthermore, mild hyperuricemia inhibits the NO system in the kidney Citation[11]. Accordingly, it has been reported that uric acid may cause microvascular disease and vasoconstriction of preglomerular vessels, which results in glomerular hypertension Citation[30]. Moreover, it has recently been shown that high levels of SUA affected endothelial function of conduit arteries in healthy adults Citation[31]. Confirming these findings, in the present study, the association of SUA to CFR impairment was independent of serum creatinine levels in both multivariate and stratified analysis.
So far, the mechanisms that may be involved in the alteration of CFR in hypertension have not been studied extensively in humans due to the complex and invasive techniques used to evaluate CFR. TTDE, a recently developed technique, is capable of measuring coronary blood flow velocity in the middle to distal portion of the LAD. Pharmacological stress TTDE is a highly reproducible tool in evaluating CFR, and its feasibility has been validated Citation[32]. Furthermore, CFR measured by TTDE has recently been shown to have an excellent correlation with CFR measured by positron emission tomography, which has been validated as a gold standard for CFR measurement Citation[33].
Several possible mechanisms could explain reduced CFR in hypertensive patients: (i) basal coronary flow may be increased due to both increased oxygen demand (increased rate‐pressure product) and myocardial mass Citation[34]; and (ii) maximal coronary flow response to physiological or pharmacological stimuli may be limited by a reduction in the overall maximal cross‐sectional area lumen of the microcirculatory bed and of intramyocardial coronary arteries Citation[35], Citation[36]. However, since coronary blood flow predominantly occurs during diastole, impairment in LV diastolic function may also play an important role in CFR impairment in hypertension Citation[22]. Moreover, it has been suggested that impairment of CFR may occur very early in hypertension before hypertrophy is apparent, and therefore may cause subsequent ischemia and fibrosis Citation[12]. LV diastolic function contributes to regulate the relation between coronary flow and pressure perfusion. The impairment of LV relaxation has shown an adverse impact on early diastolic coronary flow. Coronary microvessel damage may not be evident at baseline, but can become overt with faster heart rate Citation[22].
In conclusion, the present study demonstrated an independent association between SUA levels and CFR impairment in untreated essential hypertensive subjects even in the absence of known CV risk factors. These results support a role for SUA level as an independent marker of target organ damage in hypertension.
Study limitations
TTDE does not measure the absolute volumetric flow in the LAD but only flow velocity; thus an unchanging vessel diameter is assumed in the assessment of coronary flow response to a vasodilator stimulus. Dipyridamole mildly dilates epicardial coronary vessels. However, the standardized pharmacological protocol of dipyridamole to measure hyperemic coronary flow and thus the CFR is still controversial, and 0.56 mg/kg dose has generally been used in most previous studies Citation[37], Citation[38]. Thus, we used low‐dose dipyridamole (0.56 mg/kg over 4 min) for CFR assessment.
In this study, we have excluded hypertensive subjects with confounding factors, which are commonly encountered in normal population such as diabetes mellitus, obesity, dyslipidemia and CAD, for both CFR and SUA levels to investigate the independent association between SUA levels and CFR. Therefore, the study does not provide information about the association between SUA levels and CFR in overall hypertensive population.
Conflict of interest disclosures (none)
Each and every author does not have any personal or financial relationships that have any potential to inappropriately influence (bias) his or her actions or manuscript, and no financial or other potential conflicts of interest exist (includes involvement with any organization with a direct financial, intellectual, or other interest in the subject of the manuscript) regarding the manuscript. In addition, there are no any grants and sources of financial support related to the topic or topics of the manuscript.
References
- Regoli F., Winston G. W. Quantification of total oxidant scavenging capacity of antioxidants for peroxynitrite, peroxyl radicals, and hydroxyl radicals. Toxicol Appl Pharmacol 1999; 156: 96–105
- Freedman D. S., Williamson D. F., Gunter E. W., Byers T. Relation of serum uric acid to mortality and ischemic heart disease. The NHANES I Epidemiologic Follow‐up Study. Am J Epidemiol 1995; 141: 637–644
- Alderman M. H. Uric acid and cardiovascular risk. Curr Opin Pharmacol 2002; 2: 126–130
- Brand F. N., McGee D. L., Kannel W. B., Stokes J., Castelli W. P. Hyperuricemia as a risk factor of coronary heart disease: The Framingham Study. Am J Epidemiol 1985; 121: 11–18
- Fang J., Alderman M. H. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow‐up study, 1971–1992. National Health and Nutrition Examination Survey. JAMA 2000; 283: 2404–2410
- Liese A. D., Hense H. W., Lowel H., Doring A., Tietze M., Keil U. Association of serum uric acid with all‐cause and cardiovascular disease mortality and incident myocardial infarction in the MONICA Augsburg cohort. World Health Organization Monitoring Trends and Determinants in Cardiovascular Diseases. Epidemiology 1999; 10: 391–397
- Alderman M. H., Cohen H., Madhavan S., Kivlighn S. Serum uric acid and cardiovascular events in successfully treated hypertensive patients. Hypertension 1999; 34: 144–150
- Lehto S., Niskanen L., Ronnemaa T., Laakso M. Serum uric acid is a strong predictor of stroke in patients with non‐insulin‐dependent diabetes mellitus. Stroke 1998; 29: 635–639
- Langlois M., De Bacquer D., Duprez D., De Buyzere M., Delanghe J., Blaton V. Serum uric acid in hypertensive patients with and without peripheral arterial disease. Atherosclerosis 2003; 168: 163–168
- Hoieggen A., Alderman M. H., Kjeldsen S. E., Julius S., Devereux R. B., De Faire U., , LIFE Study Group, et al. The impact of serum uric acid on cardiovascular outcomes in the LIFE study. Kidney Int 2004; 65: 1041–1049
- Johnson R. J., Kang D. H., Feig D., Kivlighn S., Kanellis J., Watanabe S., et al. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease?. Hypertension 2003; 41: 1183–1190
- Laine H., Raitakari O. T., Niinikoski H., Pitkanen O. P., Iida H., Viikari J., et al. Early impairment of coronary flow reserve in young men with borderline hypertension. J Am Coll Cardiol 1998; 32: 147–153
- Schwartzkopff B., Motz W., Frenzel H., Vogt M., Knauer S., Strauer B. E. Structural and functional alterations of the intramyocardial coronary arterioles in patients with arterial hypertension. Circulation 1993; 88: 993–1003
- Frohlich E. D. Fibrosis and ischemia: The real risks in hypertensive heart disease. Am J Hypertens 2001; 14: 194S–199S
- Brilla C. G., Janicki J. S., Weber K. T. Impaired diastolic function and coronary reserve in genetic hypertension. Role of interstitial fibrosis and medial thickening of intramyocardial coronary arteries. Circ Res 1991; 69: 107–115
- Jennings G. L., Dilley R. J. Left ventricular remodelling impacts on coronary flow reserve in hypertensive patients: Is there a vascular mechanism?. J Hypertens 2002; 20: 1291–1293
- Viazzi F., Parodi D., Leoncini G., Parodi A., Falqui V., Ratto E., et al. Serum uric acid and target organ damage in primary hypertension. Hypertension 2005; 45: 991–996
- Erhardt J. G., Estes J. E., Pfeiffer C. M., Biesalski H. K., Craft N. E. Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C‐reactive protein by an inexpensive, sensitive, and simple sandwich enzyme‐linked immunosorbent assay technique. J Nutr 2004; 134: 3127–3132
- Devereux R. B., Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation 1977; 55: 613–618
- Erdogan D., Gullu H., Caliskan M., Yildirim I., Ulus T., Bilgi M., et al. Coronary flow reserve in dipper and non‐dipper hypertensive patients. Blood Press 2005; 14: 345–352
- Caiati C., Montaldo C., Zedda N., Bina A., Iliceto S. New noninvasive method for coronary flow reserve assessment: Contrast‐enhanced transthoracic second harmonic echo Doppler. Circulation 1999; 99: 771–778
- Galderisi M., Cicala S., Caso P., De Simone L., D'Errico A., Petrocelli A., et al. Coronary flow reserve and myocardial diastolic dysfunction in arterial hypertension. Am J Cardiol 2002; 90: 860–864
- Fessel W. J. High uric acid as an indicator of cardiovascular disease. Independence from obesity. Am J Med 1980; 68: 401–404
- Campion E. W., Glynn R. J., DeLabry L. O. Asymptomatic hyperuricemia. Risks and consequences in the normative aging study. Am J Med 1987; 82: 421–426
- Ward H. J. Uric acid as an independent risk factor in the treatment of hypertension. Lancet 1998; 352: 670–671
- Messerli F. H., Frohlich E. D., Dreslinski G. R., Suarez D. H., Aristimuno G. G. Serum uric acid in essential hypertension: An indicator of renal vascular involvement. Ann Intern Med 1980; 93: 817–821
- Verdecchia P., Schillaci G., Reboldi G., Santeusanio F., Porcellati C., Brunetti P. Relation between serum uric acid and risk of cardiovascular disease in essential hypertension. The PIUMA study. Hypertension 2000; 36: 1072–1078
- Mattei P., Arzilli F., Giovannetti R., Penno G., Arrighi P., Taddei S., et al. Microalbuminuria and renal haemodynamics in essential hypertension. Eur J Clin Invest 1997; 27: 755–760
- Khosla U. M., Zharikov S., Finch J. L., Nakagawa T., Roncal C., Mu W., et al. Hyperuricemia induces endothelial dysfunction. Kidney Int 2005; 67: 1739–1742
- Sanchez‐Lozada L. G., Tapia E., Santamaria J., Avila‐Casado C., Soto V., Nepomuceno T., et al. Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int 2005; 67: 237–247
- Erdogan D., Gullu H., Caliskan M., Yildirim E., Bilgi M., Ulus T., et al. Relationship of serum uric acid to measures of endothelial function and atherosclerosis in healthy adults. Int J Clin Pract 2005; 59: 1276–1282
- Caiati C., Zedda N., Montaldo C., Montisci R., Iliceto S. Contrast‐enhanced transthoracic second harmonic echo Doppler with adenosine: A noninvasive, rapid and effective method for coronary flow reserve assessment. J Am Coll Cardiol 1999; 34: 122–130
- Saraste M., Koskenvuo J., Knuuti J., Toikka J., Laine H., Niemi P., et al. Coronary flow reserve: Measurement with transthoracic Doppler echocardiography is reproducible and comparable with positron emission tomography. Clin Physiol 2001; 21: 114–122
- Kozakova M., Palombo C., Pratali L., Pittella G., Galetta F., L'Abbate A. Mechanisms of coronary flow reserve impairment in human hypertension. An integrated approach by transthoracic and transesophageal echocardiography. Hypertension 1997; 29: 551–559
- Opherk D., Mall G., Zebe H., Schwarz F., Weihe E., Manthey J., Kubler W. Reduction of coronary reserve: A mechanism for angina pectoris in patients with arterial hypertension and normal coronary arteries. Circulation 1984; 69: 1–7
- Treasure C. B., Klein J. L., Vita J. A., Manoukian S. V., Renwick G. H., Selwyn A. P., et al. Hypertension and left ventricular hypertrophy are associated with impaired endothelium‐mediated relaxation in human coronary resistance vessels. Circulation 1993; 87: 86–93
- Wilson R. F., Laughlin D. E., Ackell P. H., Chilian W. M., Holida M. D., Hartley C. J., et al. Transluminal subselective measurement of coronary blood flow velocity and vasodilator reserve in man. Circulation 1985; 72: 82–92
- Galderisi M., Cicala S., D'Errico A., de Divitiis O., de Simone G. Nebivolol improves coronary flow reserve in hypertensive patients without coronary heart disease. J Hypertens 2004; 22: 2201–2208