Abstract
Objective. Both arterial stiffness and proteinuria are important markers for organ damage in hypertension. This study was planed to investigate the association between arterial stiffness and inflammation and to define the influences of proteinuria on arterial stiffness and inflammation in non‐diabetic hypertension. Methods. We enrolled 205 patients (mean age 41±8 years, 66 women) with essential hypertension noted for less than 5 years in this study. They did not have diabetes mellitus or any overt cardiac, vascular, or renal complications. Stiffness index (SI) derived from digital volume pulse was used for assessment of arterial stiffness. High‐sensitivity C‐reactive protein (hsCRP) was measured in each patient during enrollment. Left ventricular hypertrophy (LVH) was documented by electrocardiography and proteinuria was assessed by measuring 24‐h urine protein. Results. SI was significantly correlated with hsCRP (r = 0.166, p = 0.017). LVH was noted in 34 patients (17%). SI was significantly higher in patients with LVH (8.03±1.74 vs 7.19±1.19 m/s, p = 0.001). Proteinuria was noted in three patients with LVH. SI was gradually increased among patients without LVH, with LVH but not proteinuria, and with LVH and proteinuria (7.19±1.19, 7.68±1.21, 11.75±2.51 m/s respectively; p<0.001). HsCRP was also gradually increased among patients without LVH, with LVH but not proteinuria, and with LVH and proteinuria (0.20±0.24, 0.30±0.59, 1.56±1.58 mg/dl respectively; p<0.001). Conclusions. SI was significantly correlated with hsCRP. Arterial stiffness and inflammation were increased in association with proteinuria in non‐diabetic essential hypertension.
Introduction
Arterial stiffness is well recognized as a prognostic factor for cardiovascular disease in hypertension Citation[1]. Among various methods for measurement of arterial stiffness, pulse wave velocity (PWV) is a popular method currently Citation[2], Citation[3]. PWV increases with age and with presence of risk factors Citation[4]. A stiffness index (SI) derived from digital volume pulse measured by photoplethysmography has been developed, and it is well correlated with PWV and age Citation[5–7]. Photoplethymography can measure digital volume pulse easily by using transmission of infrared light through a finger pad Citation[8]. Our previous study has demonstrated that SI derived from digital volume pulse was associated with left ventricular mass measured by echocardiography, renal dysfunction, and vascular disease in patients with untreated hypertension Citation[9]. It could be an independent marker for overt target organ damage in hypertension Citation[9].
Inflammation plays an important role in atherosclerosis. Elevation of C‐reactive protein (CRP) is associated with increased cardiovascular risk Citation[10]. Increased levels of CRP have been shown to be associated with endothelial dysfunction Citation[11] and with increased pulse pressure, a surrogate marker for arterial stiffness of large vessels Citation[12]. CRP levels were noted to be higher in subjects with hypertension Citation[13]. Relationship between CRP and arterial stiffness in hypertension was not well defined before. Some studies showed that CRP was correlated with arterial stiffness but some studies were not Citation[14–17].
In order to define the relationship between SI and inflammation in hypertension, we enrolled 205 uncomplicated hypertensive patients in this study. We measured serum high‐sensitivity CRP (hsCRP) in these patients to study their relationship with SI. We also measured 24‐h urinary protein to study the influences of proteinuria on arterial stiffness and hsCRP.
Methods
Subjects
Two hundred and five consecutive patients (mean age 41±8 years, 139 men) with uncomplicated essential hypertension from a hypertension clinic were included in this study. Eight‐five percent of patients were treated with antihypertensive agents. Blood pressure was measured using the standard sphygmomanometry method used in clinic. The patients were measured in sitting position after at least 5 min of resting. Hypertension was diagnosed if blood pressure was more than 140/90 mmHg on two separate occasions. All patients received the appropriate evaluation to exclude secondary hypertension. Patients with diabetes, duration of hypertension >5 years, overt cardiac disease, vascular disease, or renal dysfunction (plasma creatinine concentration >1.5 mg/dl) were excluded. Informed consent was obtained in each subject. The study protocols were approved by ethic committee in our medical center.
Acquisition of SI
Digital volume pulse was measured in the right index finger by photoplethysmography (Micro Medical, Gillingham, UK) transmitting infrared light at 940 nm. Frequency response of the photoplethysmography was flat to 10 Hz. Digital output from the photoplethymsography was recorded through an analogue‐to‐digital converter (12 bits, sampling frequency 100 Hz). The digital volume pulse waveforms were recorded for 10 s, and SI was calculated automatically by computer as body height (m) divided by transition time (s) from the first systolic peak to the inflection point of the reflection wave Citation[9]. All measurements were made with the subject supine in a temperature‐controlled laboratory at 25±1°C. All subjects were allowed to acclimatize to this temperature for at least 10 min before recordings commenced Citation[7].
Measurement of hsCRP
Serum hsCRP was measured using latex‐enhanced immunonephelometric assays on a BN II analyzer (Dade Behring, Deerfield, IL, USA), which we have reported before Citation[18].
Detection of left ventricular hypertrophy (LVH)
Standard 12‐lead electrocardiography was used for detection of LVH. Presence of LVH was defined by Sokolow–Lyon voltage criteria. LVH was present when summation of amplitude of R wave in V1 and S wave in V5 or V6 was ⩾35 mm Citation[19].
Detection of proteinuria
For measurements of total urinary protein, 24‐h urine samples were used. Clinical proteinuria was considered if total urinary protein ⩾300 mg in the 24‐h urine sample Citation[20].
Statistical analysis
The relations between SI and age, blood pressure, hsCRP, and lipid profiles were assessed using Pearson's correlation. Differences between patients with or without LVH were compared with Student's t‐test, for continuous variables, or the chi‐square test, for categorical variables. Multiple logistic regression analysis was used for assessment of the most independent factor for occult organ damage. All data are presented as the mean±standard deviation (SD). A p‐value of less than 0.05 was considered statistically significant. All analysis was performed with SPSS 11.5 for Windows (SPSS Institute, Chicago, IL, USA).
Results
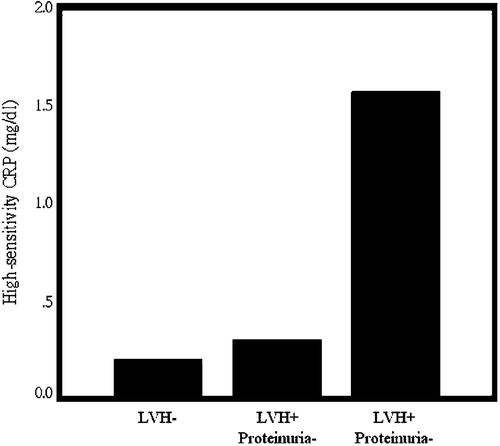
SI was significantly correlated with age, diastolic blood pressure, mean blood pressure, high‐density lipoprotein, hsCRP, and 24‐h urinary protein (Table ). Total urinary protein was significantly correlated with hsCRP (r = 0.562, p<0.001). There were 35 patients (17%) with LVH. SI and hsCRP were significantly higher in patients with LVH. Lipid profiles, blood sugar, age, blood pressure, sex, smoking, history of hyperlipidemia, and type of medications did not affect occurrence of LVH (Table ). All of the patients with proteinuria had LVH. SI was gradually increased among patients without LVH, with LVH but not proteinuria, and with LVH and proteinuria (7.19±1.19, 7.68±1.21, 11.75±2.51 m/s respectively, p<0.001) (Figure ). HsCRP was also gradually increased among patients without LVH, with LVH but not proteinuria, and with LVH and proteinuria (0.20±0.24, 0.30±0.59, 1.56±1.58 mg/dl respectively; p<0.001) (Figure ). Multiple logistic regression analysis revealed that SI, hsCRP, and mean blood pressure were independent factors associated with occult target organ damage (either LVH, or proteinuria) (Table ).
Table I. Correlation of stiffness index with clinical characteristics.
Table II. Comparison between patients with and without left ventricular hypertrophy.
Table III. Multiple logistic regression analysis for factors associated with organ damage.
Figure 1 Stiffness index(SI) was stepwise increased in patients without left ventricular hypertrophy (LVH−), with left ventricular hypertrophy but no proteinuria (LVH+, proteinuria−), and with both left ventricular hypertrophy and proteinuria (LVH+, proteinuria+) (p<0.001 by one‐way analysis of variance).
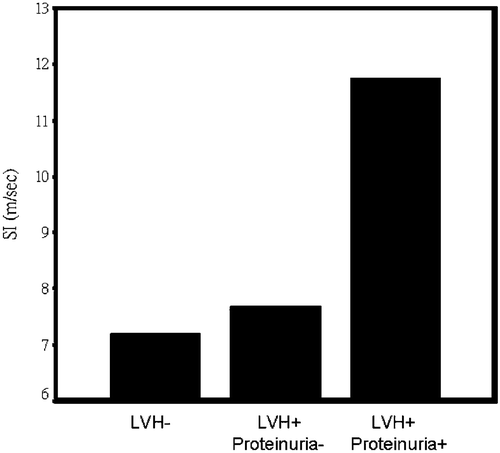
Figure 2 High‐sensitivity C‐reactive protein (hsCRP) was stepwise increased in patients without left ventricular hypertrophy (LVH−), with left ventricular hypertrophy but no proteinuria (LVH+, proteinuria−), and with both left ventricular hypertrophy and proteinuria (LVH+, proteinuria+) (p<0.001 by one‐way analysis of variance).
Discussion
The present study showed that SI derived from digital volume pulse was an independent factor associated with early organ damage in hypertension. Plasma hsCRP was also an independent factor for organ damage in hypertension, and it was correlated with SI. Our previous study demonstrated the usefulness of SI derived from digital volume pulse in evaluating overt renal or vascular damage in hypertension Citation[9]. Results from our present study have further extended the clinical significance of SI to evaluation of early occult organ damage in hypertension. SI is a simple surrogate marker for the target organ damage of hypertension.
LVH is an important independent cardiovascular risk factor in hypertension Citation[21]. Our previous study had already demonstrated that SI from digital volume pulse was correlated with left ventricular mass index assessed by M‐mode echocardiography Citation[9]. In the present study, we used electrocardiography to detect the presence of LVH and demonstrated that SI was increased in association with LVH. Although the specificity for LVH is lower in electrocardiography, it is still a simple and useful method for early detection of LVH in hypertension Citation[19]. Both electrocardiography and photoplethysmography are easy‐to‐use tools and available for risk stratification in a large population. Increased SI suggests increased PWV and arterial stiffness Citation[6], which results in increased impedance of cardiac output. The development of LVH is probably due to this hemodynamic overload of the heart Citation[22]. In a recent study, left ventricular mass index was correlated with radial augmentation index Citation[22]. Since digital volume pulse by a transfer function of photoplethysmography was similar to radial pulse detected by tonometry Citation[5], increased SI in digital volume pulse also implied enhanced wave refection might be related to the development of LVH Citation[22].
Our study showed that SI was more correlated to diastolic blood pressure and mean blood pressure than systolic blood pressure and pulse pressure. This finding did not agree with other reports Citation[4], Citation[8], Citation[14]. Although the reasons for this discrepancy were not clear, some possible differences could be identified between our study and others. One of the possible reasons was that SI in our study was related to the measurement of peripheral instead of central arteries. It is well known that the difference between central and peripheral blood pressure is much bigger for systolic compared to diastolic blood pressure. Another possible reason for the discrepancy was that we included relative young patients with minimal atherosclerosis. Influences of systolic blood pressure and pulse pressure would not be high in this group of patients. Furthermore, good and steady digital volume pulse was necessary for the measurement of SI. Patients with deformed fingers, arrhythmias, or peripheral occlusive artery diseases were difficult to detect Citation[9]. Some selection bias could be also present in this study.
Our study also demonstrated that SI and hsCRP were increased in association with proteinuria. Proteinuria is a major risk factor for cardiovascular disease in diabetic patients Citation[20]. Increased aortic PWV was noted to be associated with microalbuminuria in type 2 diabetes Citation[23]. Proteinuria was also an adverse factor for cardiovascular disease in non‐diabetic hypertension Citation[20]. Urinary albumin excretion was associated with subclinical atherosclerosis both in diabetic and non‐diabetic populations Citation[24]. Microalbuminuria was noted to be associated with abnormal aortic mechanics assessed by m‐mode echocardiography in essential hypertensive patients Citation[25]. PWV was also noted higher in hypertensive patients with microalbuminuria, and levels of PWV had a tight correlation with albumin excretion rate Citation[26]. These results were consisted with our findings. Arterial stiffness is correlated with occult renal damages in essential hypertension. Although we used SI derived from digital volume pulse to assess arterial stiffness, our data still demonstrated closed relation between arterial stiffness and renal damage. Proteinuria was associated with increased SI and hsCRP in our study. Presence of proteinuria is also an important risk factor for cardiovascular diseases in non‐diabetic hypertension.
Our present study demonstrated that hsCRP was independently correlated with occult organ damage in hypertension. Several studies have found that systemic inflammation was associated with LVH Citation[27] or microalbuminuria Citation[28], Citation[29] in hypertension. Levels of hsCRP were correlated with both SI and urinary protein in our study. We believe that the relationship between SI and organ damage was partially through the influences of systemic inflammation, although both SI and hsCRP were independent factors for the presence of occult organ damage in our study. Elevation of hsCRP was associated with endothelial dysfunction Citation[11]. Glomerular endothelial dysfunction was thought to be a major mechanism of microalbuminuria and development of proteinuria Citation[20]. Endothelial function also plays a major role in development of arterial stiffness Citation[30]. Arterial stiffness is related to systemic inflammation not only in hypertensive patients Citation[31] but also in general population Citation[32]. Relationship between blood pressure and microalbuminuria was modified by CRP Citation[29], and left ventricular concentric remodeling was also affected by systemic inflammation in hypertension Citation[33].
One of our limitations was that we included both treated and untreated hypertensive patients in this study. The effects of antihypertensive agents on SI, hsCRP, and organ damage could not be well evaluated. However, our study demonstrated that different classes of anti‐hypertensive medications did not affect our measurements. SI and hsCRP were still independent factors for occurrence of occult organ damage after multivariate analysis. Another limitation was that we used total urine protein instead of measurement of urinary albumin excretion. According to previous reports, proteinuria is still useful in predicting cardiovascular disease in hypertension Citation[20]. Although occurrences of proteinuria were low in this study, our results still demonstrated that proteinuria had determinant effects on arterial stiffness and inflammation in essential hypertension.
In conclusion, our study demonstrated that SI was correlated with inflammation in hypertension. Presence of proteinuria was strongly associated with increased arterial stiffness and inflammation in non‐diabetic hypertension.
Acknowledgement
This study was supported by Grant NSC 94‐2314‐B‐006‐113 from the National Science Council, Executive Yuan, Taipei, Taiwan and Grants NCKUH‐94‐007 and NCKUH‐95‐050 from National Cheng Kung University Hospital, Tainan, Taiwan.
References
- Laurent S., Boutouyrie P., Asmar R., Gautier I., Laloux B., Guize L., et al. Aortic stiffness is an independent predictor of all‐cause and cardiovascular mortality in hypertensive patients. Hypertension 2001; 37: 1236–1241
- Bramwell J. C., Hill A. V. Velocity of transmission of the pulse‐wave and elasticity of the arteries. Lancet 1992; i: 891–892
- Blacher J., Asmar R., Djane S., London G. M., Safar M. E. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension 1999; 33: 1111–1117
- Tsai W. C., Chen J. Y., Wang M. C., Wu H. T., Chi C. K., Chen Y. K., et al. Association of risk factors with increased pulse wave velocity detected by a novel method using dual‐channel photoplethysmography. Am J Hypertens 2005; 18: 1118–1122
- Millasseau S. C., Guigui F. G., Kelly R. P., Prasad K., Cockcroft J. R., Ritter J. M., et al. Non‐invasive assessment of the digital volume pulse: Comparison with the peripheral pulse. Hypertension 2000; 36: 952–956
- Millasseau S. C., Kelly R. P., Ritter J. M., Chowienczyk P. J. Determination of age‐related increases in large artery stiffness by digital volume contour analysis. Clin Sci 2002; 103: 371–377
- Chowienczyk P. J., Kelly R. P., MacCallum H., Millasseau S. C., Andersson T. L. G., Gosling R. G., et al. Photoplethysmographic assessment of pulse wave reflection. J Am Coll Cardiol 1999; 34: 2007–2114
- Greenwald S. E. Pulse pressure and arterial elasticity. Q J Med 2002; 95: 107–112
- Chen J. Y., Tsai W. C., Lin C. C., Huang Y. Y., Hsu C. C., Liu P. Y., et al. Stiffness index derived from digital volume pulse as a marker of target organ damage in untreated hypertension. Blood Press 2005; 14: 233–237
- Bassuk S. S., Rifai N., Ridker P. M. High‐sensitive C‐reactive protein. Curr Probl Cardiol 2004; 29: 439–493
- Fichtlscherer S., Rosenberger G., Walter D. H., Breuer S., Dimmeler S., Zeiher A. M. Elevated C‐reactive protein levels and impaired endothelial vasoreactivity in patients with coronary artery disease. Circulation 2000; 102: 1000–1006
- Abramson J. L., Vaccarino V. Relationship between physical activity and inflammation among apparently healthy middle‐aged and older US adults. Arch Intern Med 2002; 162: 1286–1292
- Bautista L. E., Atwood J. E., O'Malley P. G., Taylor A. J. Association between C‐reactive protein and hypertension in healthy middle‐aged men and women. Coron Artery Dis 2004; 15: 331–336
- Kullo I. J., Seward J. B., Bailey K. R., Bielak L. F., Grossardt B. R., Sheedy P. F., et al. C‐reactive protein is related to arterial wave reflection and stiffness in asymptomatic subjects from the community. Am J Hypertens 2005; 18: 1123–1129
- Yasmin., McEniery C. M., Wallace S., Mackenzie I. S., Cockcroft J. R., Wilkinson I. B. C‐reactive protein is associated with arterial stiffness in appearently healthy individuals. Arterioscler Thromb Vasc Biol 2004; 24: 969–974
- Kampus P., Kals J., Ristimae T., Fischer K., Zilmer M., Teesalu R. High‐sensitivity C‐reactive protein affects central hemodynamics and augmentation index in apparently healthy persons. J Hypertens 2004; 22: 1133–1139
- Amar J., Ruidavets J. B., Sollier C. B., Bongard V., Boccalon H., Chamontin B., et al. Relationship between C reactive protein and pulse pressure is not mediated by atherosclerosis or arterial stiffness. J Hypertens 2004; 22: 349–355
- Tsai W. C., Li Y. H., Lin C. C., Chao T. H., Chen J. H. Effects of oxidative stress on endothelial function after a high‐fat meal. Clin Sci 2004; 106: 315–319
- Bayes de Luna A. Clinical electrocardiography: A textbook 9. 1993; 450, Mt. Kisco, New York
- Sarnak M. J., Levey A. S., Schoolwerth A. C., Coresh J., Culleton B., Hamm L. L., et al. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation 2003; 108: 2154–2169
- Vakili B. A., Okin P. M., Devereux R. B. Prognostic implication of left ventricular hypertrophy. Am Heart J 2001; 141: 334–341
- Hashimoto J., Watabe D., Hatanaka R., Hanasawa T., Metoki H., Asayama K., et al. Enhanced radial late systolic pressure augmentation in hypertensive patients with left ventricular hypertrophy. Am J Hypertens 2006; 19: 27–32
- Smith A., Karalliedde J., De Angekis L., Goldmith D., Viberti G. Aortic pulse wave velocity and albuminuria in patients with type 2 diabetes. J Am Soc Nephrol 2005; 16: 1069–1075
- Kramer H., Jacobs D. R., Bild D., Post W., Saad M. F., Detrano R., et al. Urine albumin excretion and subclinical cardiovascular disease: The multi‐ethnic study of atherosclerosis. Hypertension 2005; 46: 38–43
- Tsioufis C. P., Lambrou S. G., Stefanadis C. I., Antoniadis D. I., Kallikazaros I. E., Pitsavos C. E., et al. Microalbuminuria is associated with abnormal thoracic aortic mechanics in essential hypertension. Am J Cardiol 2000; 86: 797–801
- Mule G., Cottone S., Vadala A., Volpe V., Mezzatesta G., Mongiovi R., et al. Relationship between albumin excretion rate and aortic stiffness in untreated essential hypertensive patients. J Intern Med 2004; 256: 22–29
- Conen D., Zeller A., Pfisterer M., Martina B. Usefulness of B‐type natriuretic peptide and C‐reactive protein in predicting of the presence or absence of left ventricular hypertrophy in patients with systemic hypertension. Am J Cardiol 2006; 97: 249–252
- Tsioufis C., Dimitriadis K., Chatzis D., Vasiliadou C., Tousoulis D., Papademetriou V., et al. Relation of microalbuminuria to adiponectin and augmented C‐reactive protein levels in men with essential hypertension. Am J Cardiol 2005; 96: 946–951
- Stuveling E. M., Bakker S. J. L., Hillege H. L., Burgerhof J. G. M., de Jong P. E., Gans R. O. B., et al. C‐reactive protein modifies the relationship between blood pressure and microalbuminuria. Hypertension 2004; 43: 791–796
- Safar M. E., London G. M., Asmar R., Frohlich E. D. Recent advances on large arteries in hypertension. Hypertension 1998; 32: 156–161
- Mahmud A., Feely J. Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension 2005; 46: 1118–1122
- Amar J., Ruidavets J. B., Bal dit Sollier C., Bongard V., Boccalon H., Chamontin B., et al. Soluble CD14 and aortic stiffness in a population‐based study. J Hypertens 2003; 21: 1869–1877
- Tsioufis C., Stougiannos P., Kakkavas A., Toutouza M., Mariolis A., Vlasseros I., et al. Relation of left ventricular concentric remodeling to levels of C‐reactive protein and serum amyloid A in patients with essential hypertension. Am J Cardiol 2005; 96: 252–256