Abstract
Background. Seasonal variation in blood pressure (BP), a usual tendency of both systolic (SBP) and diastolic BP (DBP) to rise during winter in hypertensive patients, may be related to the higher cardiovascular mortality in winter. However, it is not yet clear what factors are relevant to the seasonal BP changes. We hypothesized that arterial stiffness is related to the BP changes between summer and winter. Methods and results. Eighty‐five elderly (>55 years) patients with essential hypertension (33 males, 64±6.0 years) were enrolled. Seasonal BP profiles over at least 2 years were studied along with arterial stiffness and clinical variables (age, gender, smoking, duration of hypertension, anti‐hypertensive medications and body mass index). Both SBP and DBP were significantly higher during winter compared with three other seasons (spring 128±10.0/79±7.3 mmHg, summer 127±9.8/78±7.1 mmHg, autumn 127±10.3/78±8.0 mmHg, winter 136±12.5/81±7.6 mmHg; SBP changes; p<0.001, DBP changes; p<0.001). There were no significant seasonal differences among spring, summer and autumn. Pulse wave velocity (PWV), a widely used clinical indicator of arterial stiffness was correlated with winter–summer differences in SBP (r = 0.272, p = 0.012), but not in DBP (r = 0.188, p = 0.085). Age, which was correlated with PWV strongly (p<0.001), was not significantly related to the seasonal changes in BP (SBP changes; p = 0.114, DBP changes; p = 0.298). No other clinical variables had significant correlation with seasonal BP changes. Multivariate regression analysis revealed that PWV is the only significant predictor for winter–summer SBP changes. Conclusions. Our results established a feasible link between arterial stiffness and seasonal BP variation. These findings may partly explain higher cardiovascular risk in patients with increased arterial stiffness.
Introduction
Seasonal changes in cardiovascular disease have been well documented, usually with an increase in winter Citation[1–3]. Acute myocardial infarction exhibits a seasonal pattern with an increased incidence during winter. Data from the US National Registry of Myocardial Infarction report approximately 53% more cases in winter than during the summer, and in‐hospital case fatality rate shows a peak of 9% in winter Citation[1]. Moreover, a winter peak in occurrence has been reported for ischemic and hemorrhagic stroke Citation[2], and rupture or dissection of aortic aneurysms as well Citation[3].
Several factors may play roles in the tendency of increased cardiovascular events in winter. Cold exposure, for example, results in an increase of sympathetic activity and blood pressure (BP) levels. Besides, a significant negative correlation between ambient temperature and BP has been reported Citation[4]. Winter is also characterized by pro‐thrombotic state. Fibrinogen levels show wide seasonal variation, increasing up to 23% during the colder months Citation[5]. Furthermore, a mild surface cooling can increase platelet and red cell count, and consequently blood viscosity Citation[6]. Probably, such an unfavorable constellation of underlying factors may play a role in the increased cardiovascular disease in winter.
Among these, augmented seasonal variation of BP is one of the most important contributing factors to increased cardiovascular events. Seasonal variation of BP has been documented in normal population Citation[7], in hypertensive patients Citation[4], Citation[8], in the elderly Citation[9], Citation[10], and in dialysis patients Citation[11]. Ambient temperature Citation[7], Citation[10], age Citation[9], body mass index (BMI) Citation[9], Citation[12], smoking Citation[13], and interdialytic body weight gain Citation[11] are reported to be related to the seasonal variation of BP. However, there is no single important affecting factor underlying the seasonal variation of BP, which is consistently observed in various studies Citation[4], Citation[7], Citation[9–13].
Recently, arterial stiffness, which can be evaluated by several measurements including pulse wave velocity (PWV), has been shown to be a strong independent predictor of cardiovascular and all‐cause mortality in patients with essential hypertensionCitation[14], and in patients with older subjects over 70 years Citation[15], as well as in patients with end‐stage renal disease on hemodialysis Citation[16]. However, underlying mechanisms between arterial stiffness and increased cardiovascular mortality are not well known. Recent studies Citation[17], Citation[18] suggested possibilities that arterial stiffness is related to the variation of BP. Moreover, BP variation is more easily observed in elderly patients, in whom arterial is known to be increased. Therefore, we hypothesized that arterial stiffness is related to augmented seasonal variation of BP, and subsequently related to increased cardiovascular events during winter.
Methods
The subjects analyzed were 85 hypertensive elderly patients (>55 years) who were on regular follow‐up at Severance Hospital, Yonsei University College of Medicine between April 2002 and March 2005. Hypertension was defined as systolic BP (SBP) >140 mmHg or diastolic BP (DBP) >90 mmHg, according to the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report Citation[19]. Definition of each four seasons is as follows; spring (March–May), summer (June–August), autumn (September–November), winter (December–February). The inclusion criteria were: (i) patients who were followed regularly every 3 months over 2 years who can show the seasonal profile of the BP; (ii) patients at age 55 years or more. Exclusion criteria were: (i) recent myocardial infarction, unstable angina, congestive heart failure, previous cerebrovascular accident, malignant debilitating disease, severe respiratory disease, renal failure (creatinine>1.4 mg/dl), anemia (hemoglobin<12 g%), secondary hypertension, hyper‐ or hypothyroidism and severe dementia; (ii) patients with any kind of changes in hypertensive medication during the follow‐up period, which can influence the seasonal profile of BP
After the patients had sat down and rested for 15 min, two consecutive readings were recorded by a mercury sphygmomanometer with a standard‐sized cuff (12×35 cm), and their mean values were calculated. The average indoor temperature was maintained by an air conditioner at 23–25°C, irrespective of outdoor environment.
Arterial stiffness was estimated by brachial‐ankle PWV (baPWV, cm/s), which was measured using a volume‐plethysmographic apparatus (from PWV/ABI, VP‐2000®; Colin, Co., Ltd., Komaki, Japan) as described previously Citation[20]. Briefly, four cuffs matched with oscillometric sensors were wrapped around the upper arms and ankles, and then the pulse volume records of the bilateral brachial and tibial arteries were monitored during a continuous deflation of the cuffs. BP of each lesion could be obtained by this oscillometric method. Electrodes of the electrocardiograph were placed on both wrists, and a microphone was placed on the left edge of the sternum to detect heart sounds. Transit time between the brachial and ankle pulse waves was automatically measured from the time delay between the feet (sharp initial systolic upstroke) of the wave at the two sites. The distance between the two recording sites of baPWV was calculated automatically using the height of the patient.
All the baPWV measurements are made after the BP were stabilized at least 2 weeks after starting or changing the antihypertensive medication, because the PWV is highly influenced by the actual BP itself. Those patients could be enrolled only if they are followed up over 2 years after starting or changing the medication. Moreover, estimation of arterial stiffness using volume‐plethysmographic apparatus was done only during the winter except for four patients. To avoid interobserver variation, one experienced examiner measured all baPWV of the analyzed patients.
Age, gender, smoking, BMI, duration of hypertension, types of anti‐hypertensive medications, laboratory data and echocardiographic data were analyzed. The statistical analysis was performed using SPSS statistical analysis program (ver. 12.0, SPSS Inc., Chicago, IL, USA). Values are expressed as mean±SD. Data were analyzed by independent t‐test, paired t‐test, correlation analysis and multivariate linear regression. A p‐value of <0.05 was considered statistically significant. The study was approved by the Ethics Committee of Yonsei University, and informed consent was obtained from all participants.
Results
Demographic data such as age, sex, smoking, duration of hypertension, body weight, BMI, and types of hypertensive medications, laboratory findings and echocardiographic data are shown in Table . Mean and SD of measured baPWV were 1589±286.2 cm/s.
Table I. The characteristics of the study group.
We took the mean of four seasonal BP profiles over at least 2 years. The results of BP measurements during the seasons are shown in Table .
Table II. Seasonal systolic blood pressure (SBP) and diastolic blood pressure (DBP): Absolute values and their differences (Δ).
The winter–summer DBP difference was 3±7.3 mmHg (p<0.001). The winter–spring DBP was 2±6.8 mmHg (p = 0.002) and the winter–autumn DBP was 3±8.0 mmHg (p<0.001). There were no significant seasonal differences between spring–autumn, summer–spring and summer–autumn DBP differences.
We analyzed the correlation of the seasonal BP changes with age, BMI and PWV. PWV was correlated with winter–summer differences in SBP changes (r = 0.272, p = 0.012) (Figure ), but not in DBP changes (r = 0.188, p = 0.085). Age, which was correlated with PWV strongly (p<0.001), was not significantly related to the seasonal changes in BP (SBP changes; p = 0.114, DBP changes; p = 0.298). While PWV showed high correlation with actual BP (SBP; r = 0.384, p<0.001, DBP; r = 0.318, p = 0.003), seasonal BP changes were not influenced by actual BP itself. No other clinical variables, including laboratory findings, duration of hypertension, types of antihypertensive medication and echocardiographic data, had a significant correlation with seasonal BP changes.
Figure 1 Correlation analysis of pulse wave velocity(PWV) with the winter‐summer systolic blood pressure (SBP) changes.
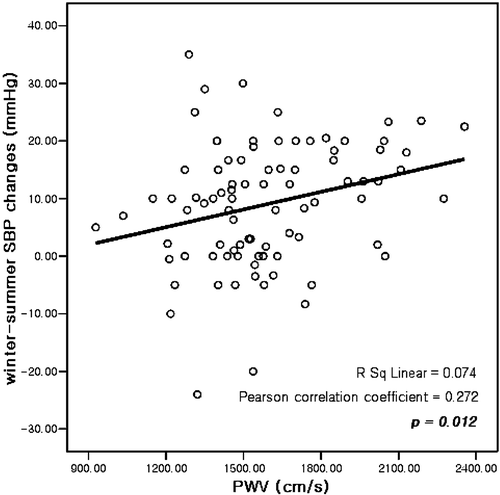
Multivariate regression analysis taking seasonal BP changes as a dependent variable revealed that PWV was the only predicting factor for seasonal SBP changes (Table ).
Table III. Multivariate regression analysis.
Discussion
We presented the relationship between arterial stiffness and seasonal BP variation in hypertensive elderly patients. Multivariate regression analysis taking SBP and DBP changes as a dependent variable with age, and BMI and PWV as an independent factor revealed that PWV was the only predicting factor for seasonal SBP changes. Our results established a feasible link between arterial stiffness and seasonal BP variation.
Seasonal variation of BP, which was previously found in various subsets of patients, is also well documented in our study group. We found both SBP and DBPs were significantly higher during winter compared with three other seasons. The degree of seasonal differences in BP that we found were similar to those reported in previous studies on hypertensives or elderly populations Citation[4], Citation[8], Citation[10].
Increased arterial stiffness was related to the winter–summer SBP changes, but not to the DBP changes in our study. These results can be explained by the inclusion criteria of over 55‐year‐old patients. SBP increases steadily with age, whereas DBP increases until about age 55 and then it declines Citation[21]. As the degree of SBP rise with aging usually outweighs the degree of DBP fall Citation[21], the relationship between arterial stiffness and seasonal BP variation can be more clearly manifested in SBP changes rather than in DBP changes.
Age Citation[9], BMI Citation[9], Citation[12] and smoking Citation[13], which were previously known to be related to the seasonal variation of BP, were not shown to be related in our study. As for the smoking, the fact that smoking results in peripheral vasoconstriction may be related with the augmented BP response in winter. However, because the number of smoker in the study group was relatively small – only two – the effects of smoking on BP variations could be masked in our results. Kristal‐Boneh et al. Citation[12] reported the inverse relationship between BMI and seasonal SBP changes, which means the augmented seasonal variation of BP in leaner patients. However, probably due to the differences of study population – lower BMI in our study group compared with previous study Citation[12] – there was no significant relationship between BMI and seasonal variation of BP.
It should be noted that age, BMI and smoking, which were known to be related to the seasonal variation of BP, are also influencing factors on the arterial stiffness itself Citation[22], Citation[23]. Even though the mechanisms of how increased arterial stiffness is accompanied by the augmented seasonal BP changes are not yet clear, there are possibilities that many of previously known influencing factors are involved in the stiffening of the arterial conduit system first, and then in the seasonal variation of BP subsequently.
Epidemiological and clinical studies have shown that increased pulse pressure is an independent cardiovascular risk factor in general population Citation[24], Citation[25]. Pulse pressure is influenced by left ventricular ejection (stroke volume and ejection time) and arterial stiffness (principally that of the aorta and large central arteries). Because left ventricular ejection remains stable or even decreases with age, arterial stiffness is the principal factor responsible for increased pulse pressure in various subsets of patients with increased cardiovascular risk Citation[24]. Recently, arterial stiffness itself, which can be evaluated by several measurements including PWV, has been shown to be a strong independent predictor of cardiovascular and all‐cause mortality in patients with essential hypertension Citation[14] and with age over 70 years old Citation[15], as well as in patients with end‐stage renal disease on hemodialysis Citation[16]. However, underlying mechanisms between arterial stiffness and increased cardiovascular mortality are not well known yet. BP variation, which was known to be related to the increased cardiovascular event Citation[26], could be the bridging point, which can explain the increased cardiovascular mortality in patients with increased arterial stiffness. Here we presented the feasible mechanism of increased cardiovascular mortality in patients with increased arterial stiffness by demonstrating the relationship between arterial stiffness and seasonal variation of BP.
These findings may give a meaningful contribution to the treatment of elderly hypertensive patients. Physicians should not overlook the BP surge during winter as an insignificant common finding. Based on our results, we can identify high‐risk patients who require more strict BP control during winter. To reduce the cardiovascular event, it is necessary to treat more carefully during winter, especially in high‐risk patients with increased arterial stiffness. Moreover, even for the normotensive patients, it is important to assess hypertension during winter, but not in other seasons, if they have increased arterial stiffness or other cardiovascular risk factors.
The present study has several potential limitations. First, our study population was relatively small. There were difficulties recruiting a large number of patients due to the relatively long follow‐up period of over 2 years and the strict exclusion criteria of our study. Second, PWV is influenced by BP for the times when PWV is measured. Even though all the PWV measurements were made after the BP was stabilized, the relationship between PWV and BP at the time of PWV measurements could not be overlooked. Third, PWV measurements using volume‐plethysmographic apparatus can pose a problem of reproducibility. However, it is well documented that intraobserver–intersession reproducibility of PWV measurements is very high, provided that the measurements are done by a single examiner Citation[27]. In this study, one experienced examiner measured all the baPWV of analyzed patients. Moreover, estimation of arterial stiffness was done only during winter except four patients. However, prospective studies in a large population would be needed to reveal more clear relationship among arterial stiffness, augmented seasonal BP variation and major adverse cardiovascular event.
In conclusion, our study of 85 elderly hypertensive patients revealed a significant increase of BP in winter. In this population, arterial stiffness, which was known as an independent cardiovascular risk factor, was found to be related to augmented seasonal variation of BP. These findings may partly explain the higher cardiovascular risk in patients with increased arterial stiffness.
References
- Spencer F. A., Goldberg R. J., Becker R. C., Gore J. M. Seasonal distribution of acute myocardial infarction in the second National Registry of Myocardial Infarction. J Am Coll Cardiol 1998; 31: 1226–1233
- Gallerani M., Portaluppi F., Maida G., Chieregato A., Calzolari F., Trapella G., et al. Circadian and circannual rhythmicity in the occurrence of subarachnoid hemorrhage. Stroke 1996; 27: 1793–1797
- Mehta R. H., Manfredini R., Hassan F., Sechtem U., Bossone E., Oh J. K., et al. Chronobiological patterns of acute aortic dissection. Circulation 2002; 106: 1110–1115
- Hata T., Ogihara T., Maruyama A., Mikami H., Nakamaru M., Naka T., et al. The seasonal variation of blood pressure in patients with essential hypertension. Clin Exp Hypertens A 1982; 4: 341–354
- Woodhouse P. R., Khaw K. T., Plummer M., Foley A., Meade T. W. Seasonal variations of plasma fibrinogen and factor VII activity in the elderly: Winter infections and death from cardiovascular disease. Lancet 1994; 343: 435–439
- Keatinge W. R., Coleshaw S. R., Cotter F., Mattock M., Murphy M., Chelliah R. Increases in platelet and red cell counts, blood viscosity, and arterial pressure during mild surface cooling: Factors in mortality from coronary and cerebral thrombosis in winter. Br Med J (Clin Res Ed) 1984; 289: 1405–1408
- Jansen P. M., Leineweber M. J., Thien T. The effect of a change in ambient temperature on blood pressure in normotensives. J Hum Hypertens 2001; 15: 113–117
- Brennan P. J., Greenberg G., Miall W. E., Thompson S. G. Seasonal variation in arterial blood pressure. Br Med J (Clin Res Ed) 1982; 285: 919–923
- Charach G., Rabinovich P. D., Weintraub M. Seasonal changes in blood pressure and frequency of related complications in elderly Israeli patients with essential hypertension. Gerontology 2004; 50: 315–321
- Woodhouse P. R., Khaw K. T., Plummer M. Seasonal variation of blood pressure and its relationship to ambient temperature in an elderly population. J Hypertens 1993; 11: 1267–1274
- Argiles A., Lorho R., Servel M. F., Chong G., Kerr P. G., Mourad G. Seasonal modifications in blood pressure are mainly related to interdialytic body weight gain in dialysis patients. Kidney Int 2004; 65: 1795–1801
- Kristal‐Boneh E., Harari G., Green M. S., Ribak J. Body mass index is associated with differential seasonal change in ambulatory blood pressure levels. Am J Hypertens 1996; 9: 1179–1185
- Kristal‐Boneh E., Harari G., Green M. S. Seasonal change in 24‐hour blood pressure and heart rate is greater among smokers than nonsmokers. Hypertension 1997; 30: 436–441
- Laurent S., Boutouyrie P., Asmar R., Gautier I., Laloux B., Guize L., et al. Aortic stiffness is an independent predictor of all‐cause and cardiovascular mortality in hypertensive patients. Hypertension 2001; 37: 1236–1241
- Meaume S., Rudnichi A., Lynch A., Bussy C., Sebban C., Benetos A., et al. Aortic pulse wave velocity as a marker of cardiovascular disease in subjects over 70 years old. J Hypertens 2001; 19: 871–877
- Blacher J., Guerin A. P., Pannier B., Marchais S. J., Safar M. E., London G. M. Impact of aortic stiffness on survival in end‐stage renal disease. Circulation 1999; 99: 2434–2439
- Murakami S., Otsuka K., Kubo Y., Shinagawa M., Matsuoka O., Yamanaka T., et al. Weekly variation of home and ambulatory blood pressure and relation between arterial stiffness and blood pressure measurements in community‐dwelling hypertensives. Clin Exp Hypertens 2005; 27: 231–239
- Ichihara A., Kaneshiro Y., Takemitsu T., Sakoda M., Hayashi M. Ambulatory blood pressure variability and brachial‐ankle pulse wave velocity in untreated hypertensive patients. J Hum Hypertens 2006; 20: 529–536
- Chobanian A. V., Bakris G. L., Black H. R., Cushman W. C., Green L. A., Izzo J. L Jr., et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA 2003; 289: 2560–2572
- Yamashina A., Tomiyama H., Takeda K., Tsuda H., Arai T., Hirose K., et al. Validity, reproducibility, and clinical significance of noninvasive brachial‐ankle pulse wave velocity measurement. Hypertens Res 2002; 25: 359–364
- Izzo J. L Jr., Shykoff B. E. Arterial stiffness: Clinical relevance, measurement, and treatment. Rev Cardiovasc Med 2001; 2: 29–34; 37–40
- Rehill N., Beck C. R., Yeo K. R., Yeo W. W. The effect of chronic tobacco smoking on arterial stiffness. Br J Clin Pharmacol 2006; 61: 767–773
- Achimastos A., Benetos A., Stergiou G., Argyraki K., Karmaniolas K., Thomas F., et al. Determinants of arterial stiffness in Greek and French hypertensive men. Blood Press 2002; 11: 218–222
- London G. M., Cohn J. N. Prognostic application of arterial stiffness: Task forces. Am J Hypertens 2002; 15: 754–758
- Fernandez‐Escribano Hernandez M., Suarez Fernandez C., Saez Vaquero T., Blanco F., Alonso Arroyo M., Rodriguez Salvanes F., et al. [Relationship between pulse pressure and clinical cardiovascular damage in elderly subjects of EPICARDIAN study.]. Rev Clin Esp 2007; 207: 284–290
- Kario K. [Blood pressure variation and cardiovascular risk in hypertension]. Nippon Rinsho 2004; 62: 2145–2156
- Matsui Y., Kario K., Ishikawa J., Eguchi K., Hoshide S., Shimada K. Reproducibility of arterial stiffness indices (pulse wave velocity and augmentation index) simultaneously assessed by automated pulse wave analysis and their associated risk factors in essential hypertensive patients. Hypertens Res 2004; 27: 851–857