Abstract
Background. Avoiding Cardiovascular events through COMbination therapy in Patients LIving with Systolic Hypertension (ACCOMPLISH) is an outcome study investigating aggressive antihypertensive combination treatment. It has achieved a larger fraction of overall patients with blood pressure (BP) <140/90 mmHg (73.3%) and diabetic patients <130/80 mmHg (43.3%) at 12 months of follow‐up than any other large outcomes trial. We have analyzed baseline predictors of BPs and BP control at 12 months. Methods. Blinded baseline and 12‐month BP was available in 10 173 patients of whom 6132 had diabetes. Univariate and multivariate logistic regression models were used for BP control at 12 months; simple and multiple regression models were used for absolute BP value at 12 months. A stepwise procedure was used to select significant predictors in multivariate analyses. Results. Mean (SD) BP fell from 145.5/80.2 mmHg (18.2/10.7 mmHg) at randomization to 132.7/74.7 mmHg (16/9.6 mmHg) at 12 months. The main baseline predictors of achieving BP control were region (USA), Caucasian race and taking lipid‐lowering drugs. The predictors of uncontrolled BP were higher baseline systolic BP values, more previous antihypertensive medications, proteinuria and previous thiazide use. Conclusion. Patients in the USA, Caucasians and patients taking lipid‐lowering therapy were most likely to reach BP targets with combination therapy. Strong predictors of uncontrolled hypertension were more severe hypertension, an established need for more antihypertensive drugs and target organ damage.
Introduction
The latest National Health and Nutrition Examination Survey (NHANES) reports that over 50 million Americans and up to 1 billion people worldwide are hypertensive.
Of these, more than 40% of patients are not receiving anti‐hypertensive treatment and of those treated, two‐thirds do not have their blood pressure (BP) controlled to the recommended goal of <140/90 mmHg Citation[1]. Since increased BP sharply increases the risk of heart disease and stroke, effective anti‐hypertensive treatment is necessary to alleviate potential long‐term cardiovascular mortality and morbidity Citation[2]. Recent guidelines have noted that the majority of patients require two or more antihypertensive medications to achieve BP control. The value of an early combination treatment approach for controlling hypertension is now being tested in a major clinical trial.
The ACCOMPLISH (Avoiding Cardiovascular events through COMbination therapy in Patients LIving with Systolic Hypertension) trial has been designed to compare the efficacy of two types of commonly used antihypertensive drug combinations in preventing major clinical outcomes in hypertensive patients at high risk of cardiovascular events. The two formulations being compared are comprised of the calcium‐channel blocker, amlodipine, combined with the angiotensin‐converting enzyme (ACE) inhibitor, benazepril; and benazepril combined with the diuretic, hydrochlorothiazide. The hypothesis for this study is to compare the calcium‐channel blocker/ACE inhibitor combination with the diuretic/ACE inhibitor combination in reducing major cardiovascular outcomes Citation[3–6]. At the same time, this trial enables an accurate estimation of the BP‐lowering efficacy of treatments based on widely used combinations of established antihypertensive agents.
Recruitment for the ACCOMPLISH trial has been completed. Of the patients enrolled, 97% were previously treated for hypertension, though only 37% of them had BP controlled when recruited to the study. Within 6 months, 73% of patients achieved their BP goal Citation[7]. This trial has provided a unique opportunity to assess predictors of the BP responses to mandated combination therapy in a large outcomes trial. Since this cohort has achieved remarkable BP control early in the trial, it has been possible to relate these hemodynamic effects to clinical and demographic characteristics of the enrolled patients. Although the treatment assignments in this study are still blinded, it is now possible to provide information of clinical interest regarding predictors of BP response.
Methods
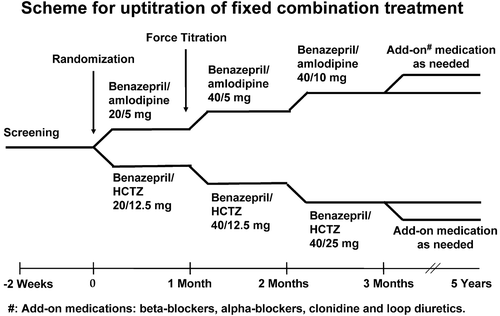
A full description of the ACCOMPLISH study outline has been published previously Citation[8]. The following is a brief description: ACCOMPLISH is a randomized, double‐blind trial that compared the efficacy of two fixed‐dose combination therapies, amlodipine besylate/benazepril and benazepril/hydrochlorothiazide, in preventing fatal and non‐fatal cardiovascular outcomes. Patients eligible for this study were >55 years with either a systolic BP (SBP) ⩾160 mmHg or currently receiving antihypertensive therapy. Eligible patients had evidence for cardiovascular, renal disease or other target organ damage. Upon study entry, patients had their study medication force‐titrated during the first 2 months of the trial to receive either amlodipine besylate/benazepril 5/40 mg or benazepril/hydrochlorothiazide 40/12.5 mg. In order to reach BP targets (SBP<140/90 mmHg), the study medication would be increased to 10/40 mg of amlodipine besylate/benazepril or 40/25 mg of benazepril/hydrochlorothiazide (). Additionally, other antihypertensive agents, excluding the drug classes involved in the trial, could be added in order to achieve target BP goals. Following the initial 3‐month period, patients were seen in 6‐month intervals until the end of the trial Citation[8].
The data in this report are based on predictor‐variable observations at baseline (the beginning of the study treatment period immediately prior to randomization). The total number of patients randomized in the trial (11 463 patients randomized to two treatment groups) reflects power calculations based on testing the principal study hypothesis for the primary cardiovascular mortality/morbidity endpoint; the details of this calculation have been published Citation[8]. A total of 10 173 patients had complete 12‐month data and were available for the present analysis in December 2006.
The primary and other mortality/morbidity endpoints of this study are not discussed in this report. We chose the 12‐month BP data for the present analysis, since the majority of patients were appropriately titrated and the study medications were stabilized by this time point.
Patient selection
The inclusion and exclusion criteria for hypertensive patients at high cardiovascular risk have been described previously Citation[8]. In general, ACCOMPLISH participants are hypertensive, aged 55 years or more, with either cardiovascular and/or renal disease and were living in the USA or Nordic area (Sweden, Norway, Denmark or Finland). Patients who were 60 years or less had evidence of two target organs damaged. Nordic‐area review boards only allowed enrollment of patients with BP not currently controlled (not a requirement for USA enrollment), and thus the baseline BP control rate was lower for Nordic countries than for the USA [21.1% vs 44.3%, respectively, with SBP/diastolic BP (DBP)<140/90 mmHg]. Mean SBP and DBP were also higher at baseline for Nordic patients (152.6/84.4 mmHg) than for US patients (142.4/78.3 mmHg).
Statistical considerations
All analyses are based on all patients with 12 months mean sitting BP data. Results are presented as mean ±SD for continuous or as n (%) for discrete variables. Thirty‐two potential baseline predictors of BP (demographic, risk and disease factors, baseline laboratory variables and prior antihypertensive medication) were identified and are listed in . The five response variables were (): SBP at 12 months, DBP at 12 months, SBP control (SBP<140 mmHg), DBP control (DBP<90 mmHg) and SBP/DBP control (SBP<140 and DBP<90 mmHg). In addition, we also modeled the sixth response variable of BP control (SBP<130 and DBP<80 mmHg) for patients with diabetes at baseline. Univariate and multivariate logistic regression models were used for BP control at 12 months, and simple and multiple linear regression models were used for absolute BP value at 12 months to evaluate baseline predictors that significantly predicted the response variables. Stepwise procedure was used to select significant (p<0.001) predictors in multivariate analyses. Missing values of the baseline predictors were imputed and replaced by median values. Where appropriate, the results are presented as χ2 with p‐values and/or odds ratio with 95% confidence intervals. The Statistical Analysis System (SAS Inc., Cary, North Carolina, USA) was used for all statistical analyses.
Table I. Potential baseline predictors and response variables of 12‐month BP.
Results
Comparison of patients controlled and not controlled at BP<140/90 mmHg
The 12‐month BPs averaged 132.7/74.7 mmHg (16.0/9.6) for all patients; 152.6/81.5 mmHg (13.2/10.1) in the patients not controlled and 125.5/72.2 mmHg (9.4/8.1 mmHg) in the patients controlled. A total of 73.3% of all patients had SBP<140 mmHg and DBP<90 mmHg at 12 months, while 74.4% had their SBP<140 mmHg and 94.0% had their DBP<90 mmHg. Of diabetic patients 73.4% had SBP<140 mmHg and DBP<90 mmHg, 74.2% had SBP<140 and 94.6% had DBP<90 mmHg. Results were similar in the overall population and in the diabetic subgroup.
Patients not controlled were particularly characterized by higher baseline BPs, serum creatinine, fasting glucose and more antihypertensive medication (). Furthermore, patients with the following characteristics were more likely to have uncontrolled BP by 12 months: being from the Nordic area, being non‐Caucasian, having prior strokes, left ventricular hypertrophy (LVH) or proteinuria, not having prior diagnosis of dyslipidemia, coronary artery disease, and not previously having revascularization. Additionally, patients treated with thiazides, beta‐blockers and insulin, and patients not treated with lipid‐lowering agents were more likely to have uncontrolled BP at 12 months ().
Table II. Baseline (continuous) variables by category, not controlled, or controlled SBP<140 and DBP<90 mmHg at 12 months.
Table III. 12‐Month control rates (SBP<140 and DBP<90) for dichotomous baseline predictor variables (demographic, medical history, prior medication use).
Comparison of patients controlled and not controlled at BP<130/80 mmHg
The 12‐month BPs averaged 132.7/74.7 mmHg (16.0/9.6 mmHg) for all patients; 141.5/78.7 mmHg (13.9/9.1 mmHg) in the patients not controlled and 119.7/68.8 mmHg (8.1/7.0 mmHg) in the patients controlled. A total of 40.3% of the entire study population had SBP<130 mmHg and DBP<80 mmHg at 12 months, 46.2% had SBP<130 mmHg, and 69.7% had DBP<80 mmHg. Of diabetic patients 43.3% had SBP<130 mmHg and DBP<90 mmHg, 48.2% had SBP<130 mmHg and 73.0% had DBP<80 mmHg.
Patients whose BP was not controlled by 12 months were characterized as having higher baseline BPs, serum creatinine, hemoglobin and more baseline antihypertensive medication (). Furthermore, patients with the following characteristics were more likely to have uncontrolled BP by 12 months: being from the Nordic area, having previous stroke, proteinuria and LVH, not previously having dyslipidemia, diabetes, coronary disease, and revascularization, prior treatment with thiazides and beta‐blockers, and no prior treatment with lipid lowering agents, oral anti‐diabetics and ACE inhibitors ().
Table IV. Baseline (continuous) variables by category, not controlled, or controlled SBP<130 and DBP<80 mmHg at 12 months.
Table V. 12‐Month control rates (SBP<130 and DBP<80 mmHg) for dichotomous baseline predictor variables (demographic, medical history, prior medication use).
Univariate predictors of 12‐month BP control
Univariate predictors of 12‐month BP<140/90 mmHg are ranked according to χ2 values in . BP control was indicated (p<0.001) by lower baseline BPs, lower glucose and serum creatinine, living in the USA, having dyslipidemia or manifestation of coronary heart disease (in particular, revascularization), LVH by electrocardiogram (ECG), not having prior proteinuria or stroke (p = 0.0002), prior use of lipid‐lowering drugs, prior treatment with fewer antihypertensive treatment classes, no prior use of thiazides, beta‐blockers (p = 0.0001) or insulin. Consistent results on univariate predictors were observed from multiple regression analysis on absolute SBP. The univariate predictors of BP above or below 130/80 mmHg in the diabetic patients were essentially the same with some variation in level of statistical significance. SBP<140 mmHg strongly followed combined <140/90 mmHg. Data are also shown for DBP<90 mmHg though this comprised 94% of the overall population and results are therefore not as reliable.
Table VI. Univariate predictors of 12‐month BP control.
Multivariate predictors of 12‐month BP control by stepwise selection
The strong (p<0.001) predictors in the multivariate analysis (full model) of 12‐month BP control <140 and <90 mmHg were similar to the significant predictors as identified from univariate analyses. The results were also similar in the multivariate analysis (full model) of 12‐month BP control <130 and <80 mmHg.
The multivariate analysis by stepwise selection (from full model, ) identified the following predictors of BP control <140 and <90 mmHg at 12 months (p<0.001) as the strongest: lower baseline SBP, region (USA), and lower numbers of antihypertensive drug classes at baseline, with the following additional significant predictors of BP control: Caucasian race, use of lipid‐lowering drugs, not previously having proteinuria and non‐use of thiazide. Similarly, for BP<130/80 mmHg in the diabetic patients, lower baseline SBP, lower numbers of previous antihypertensive drug classes, region (USA), Caucasian race, and previous coronary revascularization were identified as significant predictors of BP control.
Table VII. Multivariate predictors (stepwise selection from full model) of BP control and absolute SBP at 12 months.
Multiple regression analysis on 12‐month absolute SBP
For multiple regression analysis on absolute SBP, the significant predictors of lower post‐baseline SBP were lower baseline BP, region (USA), Caucasian race, non‐use of thiazide medication at baseline, lower numbers of previous antihypertensive drugs, no proteinuria and no LVH (by ECG) at baseline, and previous use of lipid‐lowering agents.
Discussion
The recruitment for ACCOMPLISH has been completed and the characteristics of the patients entering this trial have been described Citation[9]. Since this is an ongoing study, the concomitant therapies reflect contemporary use of drugs designed to reduce cardiovascular risk. Accordingly, ACCOMPLISH participants had high utilization of ACE inhibitors or angiotensin receptor blockers (ARBs) (52% and 26%), statins (67%) and anti‐platelet therapy (63%) at entry into the trial. The mean baseline low‐density lipoprotein‐cholesterol was 102 mg/dl while high‐density lipoprotein was 50 mg/dl. Virtually all (97% of subjects) were previously treated for hypertension (74% on two or more drugs) at study entry Citation[9].
The baseline features of the cohort indicate that prescribing clinicians are more commonly adopting aggressive treatment strategies. Even so, the overall baseline BP control rate was only 37%. Taken together with the observation that many ACCOMPLISH subjects are obese (average body mass index of 31 kg/m2) and that 61% are diabetic, it is possible that these secular trends in obesity and DM could create a population more resistant to traditional strategies for BP control. The traditional approach to hypertension management has been to initiate monotherapy and then sequentially add further medications as needed to achieve a target BP goal Citation[1]. However, treatment with initial combination therapy has been reported to result in prompt and robust reductions in BP Citation[10]. So the ACCOMPLISH trial is characterized by a larger fraction of all patients with BP<140/90 mmHg (73.3%), or diabetic patients with BP<130/80 mmHg (43.3%), at 12 months of treatment than any previous major hypertension outcomes trial. This provides an opportunity to investigate what clinical characteristics might determine BP responses to drug therapy.
The treatment regimens used in this trial have reduced the BP in a large cohort of high‐risk hypertensive patients, many obese and diabetic, to 133/75 mmHg by 12 months. The mean BP immediately prior to the start of study treatment was already at a relatively low level of 146/80 mmHg, indicating that almost all patients entering this trial (97%) were previously receiving active treatment, most often with multiple drugs (74%), for their hypertension Citation[9].
It has been demonstrated that starting treatment with combination therapy is a more effective strategy for BP management than starting with single agents Citation[5], Citation[11], Citation[12]. A further contributor to the high response rates observed in this trial was the forced titration of the combination drug regimens from an initial to an intermediate dose level. The rationale for this forced titration was primarily to ensure that patients in the two treatment arms received similar doses of the ACE inhibitor without regard to levels of achieved BP Citation[8]. Our findings indicate that for a meaningful proportion of high‐risk hypertensive patients it is possible to achieve BP values substantially below the levels currently recommended as targets by published hypertension guidelines without undue risk of hypotension or treatment discontinuation Citation[1], Citation[13].
The goal BP of <140/90 mmHg has so far been achieved in 73% of the patients in this study. Of note, this target was achieved similarly in men and women and for the most part independent of age, obesity and diabetes status. In the US cohort, control rates improved from 44% (consistent with NHANES) Citation[14] to >78%, a result which is unprecedented in clinical trials. BP control was also substantially improved for Nordic patients: from 21% at baseline to 62% at 12 months. Previous BP studies had indicated that treatment with antihypertensive drug combinations with complementary actions, including blockers of the renin–angiotensin system paired with either diuretics or calcium‐channel blockers, would work equally well across most demographic hypertensive groups Citation[15], Citation[16].
Emphasis should be placed on the BP results in diabetic patients, since they comprise 61% of the study cohort. When judged by the criteria of <140/90 or <130/80 mmHg, these patients have control rates similar to the overall cohort. In keeping with contemporary hypertension guidelines, the protocol for this study had recommended that BPs in patients with diabetes or chronic kidney disease be reduced to a target of <130/80 mmHg Citation[1], Citation[13]. This goal, however, was not a mandatory requirement of the protocol, and so it is not entirely surprising that the control rate in diabetic patients judged by this stringent BP level (<130/80 mmHg) was 43%. Despite the requirements of the study protocol, some investigators might have made judgments based on factors such as treatment side‐effects or concomitant medical conditions to withhold or delay the use of more aggressive antihypertensive therapies. It is likely, as the trial progresses, that a growing proportion of the non‐controlled patients will have their treatment regimens advanced. This has happened in other trials Citation[2], Citation[17–26].
Though high BP control rates were achieved in all patient subgroups, the univariate analysis for patients achieving a BP<140/90 mmHg identified seven key predictors. These were: being treated in the USA; taking lipid‐lowering therapy; not having LVH by ECG; having lower baseline BPs; being treated primarily with fewer antihypertensive drug classes; and not taking thiazides at baseline. Multivariate predictor analyses for achieving a BP<140/90 mmHg by stepwise selection identified seven significant variables. These were similar to those identified in the univariate analysis, except that LVH and baseline DBP were now replaced as predictors of good response by two other factors: being Caucasian and not having proteinuria.
The higher likelihood of reaching BP targets in American subjects has been previously described in hypertension trials Citation[2], Citation[27], Citation[28]. However, since lower baseline SBP values were also strong indicators of subsequent BP control, it is likely that the lower baseline BP values in US patients in this trial could explain their lower achieved BP values. Since it is reasonable to assume that Nordic investigators were as diligent as their American counterparts in striving to achieve BP goals, we conclude that these were true differences between the Nordic and American patients.
In fact, unlike the US patients, those in the Nordic countries could enter the study only if they had failed to achieve BP control on previous therapy. Notably, despite access to high doses of effective treatments, the Nordic patients as a group never caught up and achieved the same BP control levels as the Americans. This finding emphasizes that patients whose BPs fail to respond well to initial antihypertensive therapies often continue to demonstrate resistance to treatment. Similar conclusions could be drawn from other clinical trials where early BP differences between treatment arms persisted for years despite the efforts of investigators Citation[5], Citation[12], Citation[18]. It will be of particular importance when ACCOMPLISH is completed to determine if the lesser control of BP in the Nordic patients translates into a higher rate of cardiovascular and renal endpoints.
It is not clear why being Caucasian predicted better BP responses to treatment. Overall, BP reductions in African Americans, for instance, did not appear to be markedly different from those in other ethnic groups Citation[7]. Moreover, combination therapy of the type used in ACCOMPLISH is generally equally effective in the black and non‐black patients Citation[29]. Nor is it clear why concomitant use of treatment for dyslipidemia should predict better BP‐lowering efficacy, though it is possible that patients threatened by multiple risk factors may be motivated to be more adherent to their therapies, thus achieving better efficacy. It will be interesting in future analyses to determine adherence to therapy of these high‐risk patients. The ASCOT trial highlighted the clinical outcomes benefits of jointly administering antihypertensive and lipid‐lowering therapies Citation[17], Citation[26].
Strong predictors of non‐controlled hypertension in the ACCOMPLISH trial are the typical markers of more severe hypertension and target organ damage. Due to the design of the study, all patients, regardless of risk status, received well‐dosed, effective early treatment. However, further efforts at BP normalization should then have been directed towards those patients with still uncontrolled hypertension or with cardiac, renal, cerebrovascular or diabetic manifestations in accordance with current hypertension guidelines Citation[1], Citation[13]. The rigorous implementation of stepwise intensified treatment is the most important message coming out of this particular analysis of ACCOMPLISH. Benefits would obviously be earlier BP control and more effective prevention of cardiovascular complications. Indeed, our current knowledge of the importance of BP control in high‐risk hypertensive patients should support a broad use of this intensified and forced strategy with combination therapy.
In conclusions, patients in the USA, Caucasians and those taking lipid‐lowering therapy are most likely to reach BP targets on combination therapy in the ACCOMPLISH Study. On the other hand, strong predictors of uncontrolled hypertension are more severe hypertension, a previously demonstrated need for multiple antihypertensive drugs and the presence of target organ damage. Further efforts for BP normalization should be directed towards these patients with cardiac, renal, cerebrovascular or diabetic manifestations.
References
- Chobanian A. V., Bakris G. L., Black H. R., Cushman W. C., Green L. A., Izzo J. L Jr., , and the National High Blood Pressure Education Program Coordinating Committee, et al. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. The JNC7 Report. JAMA 2003; 289: 2560–2572
- McMurray M. The health economics of the treatment of hyperlipidemia and hypertension. Am J Hypertens 1999; 12: 99S–104S
- Hansson L., Zanchetti A., Carruthers S. G., Dahlöf B., Elmfeldt D., Julius S., , for the HOT Study Group, et al. Effects of intensive blood‐pressure lowering and low‐dose aspirin in patients with hypertension: Principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet 1998; 351: 1755–1762
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317: 703–713
- ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs. diuretic: The Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002; 288: 2981–2997
- Black H. R. Optimal blood pressure: How low should we go?. Am J Hypertens 1999; 12: 113S–120S
- Jamerson K., Bakris G. L., Dahlöf B., Pitt B., Velazquez E., Gupte J., et al. Exceptional early blood pressure control rates: The ACCOMPLISH Trial. Blood Press 2007; 16: 80–86
- Jamerson K. A., Bakris G. L., Wun C. C., Dahlöf B., Lefkowitz M., Manfreda S., et al. Rationale and design of the Avoiding Cardiovascular events through COMbination therapy in Patients LIving with Systolic Hypertension (ACCOMPLISH) Trial. Am J Hypertens 2004; 17: 793–801
- Weber M. A., Bakris G. L., Dahlöf B., Pitt B., Velazquez E., Gupte J., et al. Baseline characteristics in the Avoiding Cardiovascular events through COMbination therapy in Patients LIving with Systolic Hypertension (ACCOMPLISH) trial: a hypertensive population at high cardiovascular risk. Blood Press 2007; 16: 13–19
- Jamerson K. A., Nwose O., Jean‐Louis L., Schofield L., Purkayastha D., Baron M. Initial angiotensin‐converting enzyme inhibitor/calcium channel blocker combination therapy achieves superior blood pressure control compared with calcium channel blocker monotherapy in patients with stage 2 hypertension. Am J Hypertens 2004; 17: 495–501
- Weber M. A. The ALLHAT Report: A case of information and misinformation. J Clin Hypertens 2003; 5: 9–13
- Julius S., Kjeldsen S. E., Weber M., Brunner H. R., Ekman S., Hansson L., , for the VALUE trial group, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: The VALUE randomized trial. Lancet 2004; 363: 2022–2031
- 2003 European Society of Hypertension – European Society of Cardiology guidelines for the Management of Arterial Hypertension. J Hypertens 2003; 21: 1011–1054
- Burt V. L., Whelton P., Roccella E. J., Brown C., Cutler J. A., Higgins M., et al. Prevalence of hypertension in the US adult population; results from the Third National Health and Nutrition Examination Survey. Hypertension 1995; 25: 305–313
- Neutel J. M., Smith D. H. G., Weber M. A. Effect of antihypertensive monotherapy and combination therapy on arterial distensibility and left ventricular mass. Am J Hypertens 2004; 17: 37–42
- White W. B., Giles T., Bakris G. L., Neutel J. M., Davidai G., Weber M. A. Measuring the efficacy of antihypertensive therapy by ambulatory blood pressure monitoring in the primary care setting. Am Heart J 2006; 151: 176–184
- Dahlöf B., Sever P. S., Poulter N. R., Wedel H., Beevers D. G., Caulfield M., , for the ASCOT Investigators, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo‐Scandinavian Cardiac Outcomes Trial‐Blood Pressure Lowering Arm (ASCOT‐BPLA): A multicentre randomized controlled trial. Lancet 2005; 366: 895–906
- Poulter N. R., Wedel H., Dahlöf B., Sever P. S., Beevers D. G., Caulfield M., et al. Role of blood pressure and other variables in the differential cardiovascular event rates noted in the Anglo‐Scandinavian Cardiac Outcomes Trial‐Blood Pressure Lowering Arm (ASCOT‐BPLA). Lancet 2005; 366: 907–913
- Hansson L., Lindholm L. H., Niskanen L., Lanke J., Hedner T., Niklason A., , for the Captopril Prevention Project (CAPPP) study group, et al. Effect of angiotensin‐converting enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: The Captopril Prevention Project (CAPPP) randomized trial. Lancet 1999; 353: 611–616
- Hansson L., Lindholm L. H., Ekbom T., Dahlöf B., Lanke J., Scherstén B., , for the STOP‐Hypertension‐2 study group, et al. Randomized trial of old and new antihypertensive drugs in elderly patients: Cardiovascular mortality and morbidity, the Swedish Trial in Old Patients with Hypertension‐2 study. Lancet 1999; 354: 1751–1756
- Hansson L., Hedner T., Lund‐Johansen P., Kjeldsen S. E., Lindholm L. H., Syvertsen J. O., , for the NORDIL Study Group, et al. Randomized trial effects of calcium antagonists compared with diuretics and β‐blockers on cardiovascular morbidity and mortality in hypertension: The Nordic Diltiazem (NORDIL) study. Lancet 2000; 356: 359–365
- Brown M. J., Palmer C. R., Castaigne A., de Leeuw P. W., Mancia G., Rosenthal T., et al. Morbidity and mortality in patients randomized to double‐blind treatment with a long‐acting calcium‐channel blocker or diuretic in the International Nifedipine GITS study: Intervention as a Goal in Hypertension Treatment (INSIGHT). Lancet 2000; 356: 365–372
- Dahlöf B., Devereux R. B., Kjeldsen S. E., Julius S., Beevers G., de Faire U., , for the LIFE study group, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): A randomized trial against atenolol. Lancet 2002; 359: 995–1003
- Cushman W. C., Ford C. E., Cutler J. A., Margolis K. L., Davis B. R., Grimm R. H., , for the ALLHAT Research Group, et al. Success and predictors of blood pressure control in diverse North American settings: The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). J Clin Hypertens 2002; 4: 393–404
- Weber M. A., Julius S., Kjeldsen S. E., Brunner H. R., Ekman S., Hansson L., et al. Blood pressure dependent and independent effects of antihypertensive treatment on clinical events in the VALUE trial. Lancet 2004; 363: 2049–2051
- Sever P. S., Dahlöf B., Poulter N. R., Wedel H., Beevers G., Caulfield M., , for the ASCOT Investigators, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower‐than‐average cholesterol concentrations, in the Angio‐Scandinavian Cardiac Outcomes Trial – Lipid lowering Arm (ASCOT‐LLA): A multicentre randomized controlled trial. Lancet 2003; 361: 1149–1158
- Kjeldsen S. E., Dahlöf B., Devereux R. B., Julius S., de Faire U., Fyhrquist F., , for the LIFE Study Group, et al. Lowering of blood pressure and predictors of response in patients with left ventricular hypertrophy. Am J Hypertens 2000; 13: 899–906
- Julius S., Kjeldsen S. E., Brunner H., Hansson L., Platt F., Ekman S., , for the Value Trial, et al. VALUE Trial: Long‐term blood pressure trends in 13,449 patients with hypertension and high cardiovascular risk. Am J Hypertens 2003; 16: 544–548
- Campo C., Segura J., Ruilope L. M. Factors influencing the systolic blood pressure response to drug therapy. J Clin Hypertens 2002; 4: 35–40