Abstract
Objectives. The sympathetic nervous system is implicated in the development and maintenance of hypertension. However, the predictive impact of arterial plasma catecholamines has never been reported. We investigated arterial catecholamines and blood pressures (BPs) prospectively over 20 years in a group of well‐characterized middle‐aged men. Methods. Fifty‐six of original 79 men were available for the follow‐up. Multiple regression analysis was done with mean BP at follow‐up as a dependent variable, and arterial plasma catecholamines and BP at baseline as independent variables. Results. Half of the originally normotensive men developed hypertension during follow‐up. There were significant differences in the screening BP values measured at baseline between the new hypertensives and the sustained normotensives. Multiple regression analysis revealed arterial adrenaline at baseline as an independent predictor of mean BP at follow‐up in the new hypertensives (β = 0.646, R2 = 0.42, p = 0.007). Furthermore, arterial noradrenaline at baseline was a negative independent predictor of mean BP at follow‐up in the sustained normotensives (β = −0.578, R2 = 0.334, p = 0.020). Noradrenaline increased with age in the group as a whole (1318±373 vs 1534±505 pmol/l, p = 0.010) while adrenaline did not change. Conclusion. Our data suggest that arterial adrenaline is involved in the development of hypertension over 20 years in middle‐aged men. Men with sustained normotension may have an inherent protection against sympathetic overactivity. Furthermore, screening BP at baseline in normotensive men differentiated between those who developed hypertension and those who remained normotensive at follow‐up.
Introduction
The sympathetic nervous system (SNS) and the renin–angiotensin–aldosterone system (RAAS) are both implicated in the development and maintenance of hypertension. These two systems interact and reinforce their actions on blood pressure (BP) both acutely and chronically, and promote hypertension‐related structural changes in the vasculature, heart and kidneys Citation[1], Citation[2]. We have previously investigated the influence of SNS activity on BP and other cardiovascular risk factors in hypertensive and normotensive subjects in different age groups Citation[3–7]. We have also explored and discussed pathophysiological aspects of hypertension, including SNS activity and RAAS in a well‐characterized group of 42‐year‐old hypertensive and normotensive men recruited from The Oslo Study Citation[8–12].
Sympathetic nervous activity and changes therein are reflected in plasma catecholamine concentrations Citation[13]. Age‐related increase has been observed in plasma noradrenaline in cross‐sectional studies Citation[14]. Plasma noradrenaline seems to increase more with age in normotensive subjects than hypertensive subjects and it has therefore been claimed that age eliminates the differences in plasma noradrenaline between hypertensive and normotensive subjects Citation[14], Citation[15]. Plasma adrenaline is elevated in hypertensives and there are little age‐related changes in adrenaline according to cross‐sectional observations Citation[14]. Age‐related increase in muscle SNS activity in hypertensive and normotensive subjects has also been reported Citation[15–20]. However, the long‐term development of sympathoadrenal activity remains mostly unknown, and data are inconsistent. Furthermore, long‐term predictive impact of arterial plasma catecholamines has never been reported. Thus, the aim of the present study was to investigate prospectively the predictive impact of arterial plasma catecholamines and BPs over 20 years in a group of well‐characterized, homogenous hypertensive and normotensive men.
Participants and methods
Participants in 1984
Seventy‐nine Caucasian men (35 hypertensive and 44 normotensive) were recruited from The Oslo Study at an age of 42.1±0.5 years. The hypertensive group (n = 35) consisted of men with untreated mild essential hypertension. They had participated in The Oslo Study with systolic BP (SBP) between 140 and 170 mmHg and/or diastolic BP (DBP) between 90 and 100 mmHg. They were included in the present study in 1984 if they had DBP between 94 and 105 mmHg Citation[11], Citation[12]. The normotensive men (n = 44) were recruited from the same population, i.e. from the normotensives of The Oslo Study. They had BP below 140/90 mmHg (Table ). BP in The Oslo Study and at the inclusion in 1984 was measured in duplicate with a mercury sphygmomanometer after 9 and 10 min recumbency. All 79 men had normal electrocardiograms (ECG), ocular fundi, urinalysis and kidney function estimated by creatinine clearance.
Table I. Characteristics of groups; original classification.
Study protocol in 1984
The study protocol in 1984 has previously been described Citation[11], Citation[12]. All participants were examined in an outpatient setting by the same physicians. They were studied at the same time of day in a quiet room and at a constant room temperature. All were familiar with clinical examination and BP recording. They fasted and abstained from smoking for 8 h and from alcohol for 24 h prior to examination. BP was recorded semi‐automatically (oscillometric technique) with an Omega 1000 Adult/pediatric (In Vivo Research Laboratories Inc., Tulsa, OK) Citation[11], Citation[12]. Short teflon catheters (Venflon, former Viggo AB, Helsingborg, Sweden) were introduced under local anaesthesia (Xylocain® without adrenaline; Astra, Södertälje, Sweden) by one investigator in the left brachial artery. None of the participants experienced complications or notable discomfort during the introduction of the catheter. The Institutional Committee of Ethics had approved the study and informed consent was obtained from each participant.
Participants in 2004
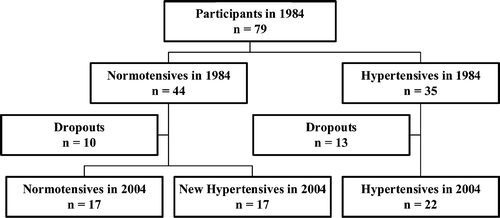
All 79 participants from 1984 were located and of these, 56 (70.1%) participated in the 20‐year follow‐up examination, 34 of the original 44 normotensive men and 22 of the original 35 hypertensive men. Seven men had died (five normotensive and two hypertensive), six men had emigrated or moved to another county (three normotensive and three hypertensive) and 10 men (two normotensive and eight hypertensive) either did not respond or responded negatively to a letter of invitation. Those who remained normotensive through the 20‐year follow‐up were defined as sustained normotensive (NTNT; n = 17). At the time of follow‐up these men had office SBP<140 and DBP<90 mmHg or mean 24‐h ambulatory SBP (ASBP) <25 mmHg and mean 24‐h DBP (ADBP) <80 mmHg. Of the 17 who remained normotensive, seven had slightly elevated office BP, but they had normal 24‐h ABP. Men defined as normotensive at baseline and who developed hypertension through the follow‐up were defined as new hypertensives (NTHT; n = 17). They had either office SBP⩾140 mmHg or office DBP⩾90 mmHg and 24‐h ASBP⩾125 mmHg and 24–h ADBP⩾80 mmHg or were taking antihypertensive medication (n = 5) at the time of follow‐up. The same criteria were used for the sustained hypertensive men (HTHT; n = 22) as for the NTHT. They were hypertensive in 1984 as well as in 2004. Among these 22 HTHT men, 16 (72.7%) used antihypertensive drugs. Of the 17 men defined as NTHTs, only five (29.4%) were taking antihypertensive drugs. Characteristics of the participants are listed in Tables and .
Table II. Characteristics of groups; present classification – NTNT, sustained normotensives; NTHT, new hypertensives; HTHT, sustained hypertensives.
Study protocol in 2004
The participants were studied in the same facilities as 20 years ago and at the same time of the day. All were familiar with clinical examination and BP measurements. They fasted and abstained from smoking for 8 h and from alcohol for 24 h prior to the examination. They were all examined by the same physicians who were not aware of their previous BP status. Arterial catheters were not inserted during the examination in 2004; blood samples were taken by venepuncture. Height, weight and body mass index (BMI) were measured and calculated using standard methods. BP was measured in duplicate with a calibrated mercury sphygmomanometer after 5 min rest in a sitting position. The arm was supported at heart level and an appropriately sized cuff was used. The mean of the two measurements was used for statistical analyses. Mean BP (MBP) was calculated using the formula: MBP = DBP+(pulse pressure/3). BP was also measured in duplicate after 30 min in supine position. Heart rate (HR) was registered after 5 min in a sitting position and after 30 min supine. Ambulatory BPs were measured with a validated oscillometric device (Spacelab 90207, SpaceLabs Inc., Redmond, WA). Readings took place every 20 min during the daytime (06:00–23:00 h) and every 60 min during the night (23:00–06:00 h). During ABP measurements, the participants were instructed to keep their arm still to ensure high‐quality recordings. According to the European Society of Hypertension guidelines, values were considered hypertensive if mean 24‐h ABP was ⩾125/80 mmHg Citation[21], Citation[22]. The participants also took their home BPs after instructions from a trained technician with a validated semiautomatic oscillometric device (Omron M4, Omron Healthcare Europe, Hoofdorp, The Netherlands) and measured BP twice after 5 min rest in a sitting position in the morning between 06:00 and 10:00 h and in the evening between 19:00 and 23:00 h on 3 consecutive days. The mean values for all home measurements were used for statistical analysis. Values were considered hypertensive if home BP was ⩾135/85 mmHg Citation[21–23]. The National Committee of Medical Research Ethics in Norway approved the follow‐up study, and concession was granted from the National Data Inspectorate in Norway. All the participants gave written informed consent.
Biochemical assays 1984 and 2004
The samples were taken as previously described Citation[11], Citation[12]. Plasma catecholamine concentrations were measured with the same radioenzymatic technique Citation[24] in 1984 and 2004; this method was validated in our laboratory and against other laboratories using the same or other methods Citation[5], Citation[25]. For noradrenaline and adrenaline, respectively, the within‐assay coefficients of variation (CV) were 8% and 14%, the day‐to‐day CV were 9% and 13%, and the between‐technician CV were 9% and 10%, in 25 samples covering the complete ranges of concentrations Citation[11]. All blood samples were analysed in a mixed order by technicians who were unaware of the participants' BP status.
Statistics
SPSS 14.0 (SPSS Inc., Chicago, IL) was used for data management and statistical analysis. Results are presented as means±standard deviations (SD) unless otherwise stated. Differences are presented as means with 95% confidence interval (CI). Parametric tests were used for normally distributed data, while nonparametric tests or log transformations of data were applied if data were skewed. Differences between two groups were assessed either by Student's t‐test or with the Mann–Whitney test and within‐group differences with either the Student's t‐test or Wilcoxon test. Comparisons between multiple groups were performed by one‐way ANOVA (analysis of variance). Univariate relation between variables were assessed either by the Spearman correlation coefficient or by the Pearson correlation coefficient, and further examined with multiple linear regression analysis using an enter procedure with assessment of co‐linear diagnostics. Multiple regression analysis was undertaken with mean office BP in 2004 at follow‐up as a dependent variable and catecholamines and BP in 1984 as independent variables. A two‐tailed p‐value <0.05 was considered statistically significant.
Results
Participants at 20‐year follow‐up in 2004
Fifty‐six of the 79 men were accessible for examination 20 years later as shown in the flowchart (Figure ). All were Caucasian of the same age; further characteristics of the subjects are given in Tables and . Six of the original 22 hypertensive men did not receive any treatment with antihypertensive drugs in 2004, and had office BP in the range 151–197/93–117 mmHg, and of those treated only one had office BP<140/90 mmHg.
BP
From the observation 20 years ago, there were three different BP measurements (Table ), the first one taken at the screening for The Oslo Study, the second one at the inclusion of the present study, and the last one taken at the time of the investigation. There was a mean difference in SBP between hypertensive and normotensive men at the time of investigation in 1984 of 17.4 mmHg (95% CI 12.7–22.0) and for DBP 14.5 mmHg (95% CI 11.1–18.1), p<0.001 for all comparisons. The change in MBP from screening to the time of investigation months later ranged from −12.7 to 18.3 mmHg, and the percentage change in MBP seemed higher in the hypertensive than in the normotensive group (5.2±7.4 vs 2.7±8.9%), but that did not reach statistical significance.
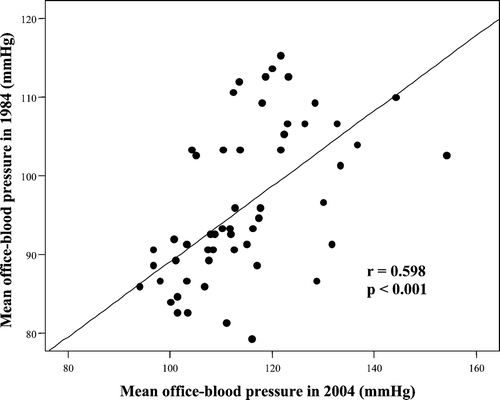
Significant differences in BP persisted between the hypertensive and normotensive groups in 2004. This was also apparent using ambulatory BP and home BP (Table ). There was a highly significant relationship between MBP in 1984 and MBP in 2004 in the group as a whole (r = 0.598, p<0.001, Figure ).
BP in HTHTs, NTNTs and NTHTs
Using the new classification (NTNTs, NTHTs, HTHTs), significant differences were found at the time of screening in 1984 in office BP between NTNTs and NTHTs (SBP, p = 0.035; DBP, p = 0.049, MBP, p = 0.048), between NTNTs and HTHTs (p<0.001 for all BP) and between NTHTs and HTHTs (p<0.001 for all BP) (Table ). At the time of inclusion and the investigation in 1984, the HTHTs had significantly higher BP than the NTNTs and NTHTs, but there was no longer significant difference in office BP between those groups (Table ).
Significant differences in office BP, 24‐h ABP and home BP were observed between all three groups in 2004 (Table ).
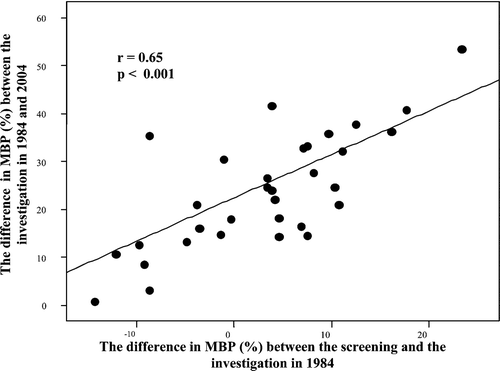
The change in supine MBP from screening time to the investigation in 1984 differed numerically between the three groups, but this difference did not reach statistical significance (−2.0, −3.5 and −5.2% for NTNTs, NTHTs and HTHTs respectively). This difference in MBP from screening to investigation was significantly related to the rise in MBP during the 20‐year follow‐up in the group as a whole (r = 0.46, p<0.001, n = 56), as well as when looking separately at the normotensive group from 1984 (r = 0.65, p<0.001, n = 34, Figure ), but not for the hypertensive group alone.
BP and plasma catecholamines
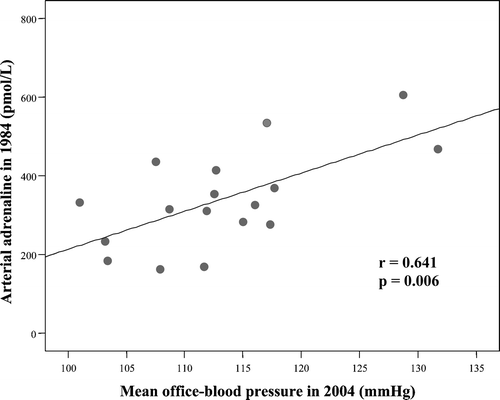
In the NTHTs, there was a significant positive relationship between arterial adrenaline in 1984 and MBP 20 years later (Figure ). Such a relationship was not observed for arterial noradrenaline in that group, while in the NTNTs a significant negative relationship between arterial noradrenaline in 1984 and MBP at follow‐up appeared. No significant relationship was found between plasma catecholamines in 1984 and MBP at follow‐up in the HTHTs.
Figure 4 Correlation between arterial adrenaline in 1984 and mean blood pressure(MBP) in 2004 in the subjects who converted from normotension to hypertension during the follow‐up, new hypertensives (NTHT; n = 17).
Overall, no association between plasma catecholamines measured in 1984 and BPs in 2004 was observed in the original normotensive and hypertensive groups.
Venous plasma noradrenaline in the whole group was significantly higher in 2004 than in 1984 (1534±505 vs 1318±373 pmol/l, p = 0.010), while venous plasma adrenaline did not change (235±152 vs 252±194 pmol/l, n.s.). Plasma noradrenaline was higher in the hypertensive than in the normotensive group in 1984 for arterial samples only (Table ). In 2004, no significant difference appeared between the groups for venous noradrenaline. Venous plasma adrenaline on the other hand differed significantly between the groups in 1984 and in 2004 (Table ).
Table III. Plasma catecholamines; original classification.
In 2004, venous plasma noradrenaline and plasma adrenaline were numerically higher in those with sustained hypertension than in the NTNTs and the NTHTs, but only borderline statistical significance was obtained for plasma adrenaline (p = 0.053). No significant differences in plasma catecholamines were observed between these three groups in 1984 (Table ).
Table IV. Plasma catecholamines; present classification – NTNT, sustained normotensives; NTHT, new hypertensives; HTHT, sustained hypertensives.
Neither arterial nor venous plasma catecholamines were related to the percentage change in MBP from screening to investigation time in 1984. A relationship between percentage difference in MBP between screening and investigation in 1984 and the percentage rise in venous plasma noradrenaline alone during the 20‐year period was found for the whole group (r = 0.27, p = 0.047, n = 56). In the combined group NTHTs and NTNTs, this relationship persisted (r = 0.36, p = 0.039, n = 33), and even in the NTHT group alone (r = 0,49, p = 0.05), but not for the NTNTs alone.
Predictors of BP after 20 years
Multiple regression analysis with MBP in 2004 as the dependent variable and catecholamines, BMI and MBP in 1984 as independent variables revealed that for the whole group, mean BP in 1984 was the only independent predictor of the BP 20 years later (β = 0.620, p<0.001, R2 = 0.384). In the NTHTs, multiple regression analysis with the same model revealed arterial adrenaline in 1984 as the only independent predictor of the mean BP 20 years later (β = 0.646, p = 0.007, R2 = 0.417), while arterial noradrenaline in 1984 was an independent negative predictor of mean BP at follow‐up in the NTNTs (β = −0.578, p = 0.020, R2 = 0.334).
Discussion
Hypertension developed in 50% of the normotensive 40‐year‐old men during the 20‐year follow‐up, and BP had also increased substantially in the hypertensive men. The men who were normotensive at baseline and developed hypertension could be identified on basis of the very first BP measurements taken in The Oslo Study in 1984. These men had slightly, yet significantly higher BP at that time albeit well within the normotensive range, compared with those who remained normotensive through the follow‐up. BPs taken under strictly standardized conditions and after 30 min in supine rest could no longer distinguish between these two groups. However, the difference in BP from screening to inclusion in 1984 was strongly related to the increase in BP over the next 20 years. Thus, the alert reaction in the BP elicited by the screening procedure seems to be reflected in the increase of BP that took place during the next two decades. We therefore suggest that the higher screening BP in 1984 in the normotensive men that converted to become hypertensive during the follow‐up could represent an increased cardiovascular reactivity to stress in that group. This could predispose to the development of hypertension as shown previously in younger men Citation[6], Citation[7], Citation[26]. In 22‐year‐old men with high screening BP, Rostrup et al. Citation[7] observed that BP became normal after 30 min of supine rest. Furthermore, these young men exhibited an increased response to stress with enhanced catecholamine responses despite similar resting plasma catecholamines as those with normal screening BP. Rostrup et al. Citation[7] hypothesized that the cardiovascular reactivity observed during screening as well as stress test could be related to increased sensitivity of adrenaline. In our study, there was no difference in plasma catecholamines measured under strictly standardized conditions between the groups who were normotensive in 1984; however, this does not exclude that differences in catecholamine response may exist. Only resting adrenaline and noradrenaline levels were measured – and a difference in catecholamine levels may only be exhibited during time of stress as shown by Rostrup et al. Citation[7] in his study of cardiovascular reactivity in young men.
In a prospective 10‐year follow‐up study in young normotensive Japanese, higher venous plasma noradrenaline levels were found in those whose BP subsequently increased more than 10% compared with those in whom BP did not rise, suggesting a role for the SNS in the initiation of hypertension Citation[27]. The same group has later published work linking serum uric acid and plasma noradrenaline to the BP elevation Citation[28]. It is not clear to us whether the changes in venous plasma noradrenaline over time were significantly different between the two groups, as there was even a difference in baseline plasma noradrenaline Citation[28]. In contrast to our original normotensive subjects who did not differ in BP at the time of investigation, the young Japanese subjects also differed significantly in both SBP and DBP with approximately 5 mmHg at the time of entry into the study, which could also explain the difference in plasma noradrenaline. Thus, differences in population, age, BP levels and blood sampling technique or the state of alertness could all contribute to the inconsistency in findings. Furthermore, differences in catecholamines between normotensives and hypertensives have been claimed to be more pronounced in younger subjects Citation[15], Citation[29]. Our study may also lend support to that assumption as a significant difference in plasma noradrenaline between the normotensive and hypertensive participants, albeit observed in arterial samples only in 1984, was not present after 20 years.
The release of adrenaline from the adrenal medulla is usually a response to increased sympathetic activity triggered by different stimuli including mental stress. The link between stress, adrenal medullary activation and the development of hypertension has been discussed previously at length by our group Citation[6], Citation[7], Citation[9], Citation[26]. The importance of sympathoadrenal activity for the development of elevated BP is substantiated by our present observation that arterial plasma adrenaline at baseline was the only independent predictor of BP after adjusting for BMI and BP levels in the men who converted from normal BP to hypertension during the follow‐up. Furthermore, venous adrenaline was clearly elevated in 1984 in the hypertensive group compared with the normotensive, and this difference actually persisted in 2004.
Age‐related changes in the sympathoadrenal system have been studied for decades Citation[10], Citation[30], Citation[31], and data are mostly based on cross‐sectional studies and with a wide age span. In the present longitudinal observations of normotensive and hypertensive men, we found a significant rise in noradrenaline during the 20 years in the group as a whole. In the same period, plasma adrenaline remained virtually unchanged. The evidence to date supports the view that ageing is associated with an activation of the SNS Citation[30]. The rise in plasma noradrenaline with age has been assumed to be due to a combination of decreased neuronal reuptake of noradrenaline and reduced metabolic clearance, and could at least partly explain the general age‐related increase in BP. The reasons for lack of change in plasma concentration of adrenaline, even though adrenaline release from the adrenal medulla decreases, have been claimed to be caused by reduced plasma clearance of adrenaline from the circulation Citation[30].
Plasma noradrenaline plays a central role in the regulation of BP, and the link between noradrenaline and BP is clearly shown by the elevated arterial noradrenaline levels in the hypertensive group in 1984 compared with the normotensive control subjects. The change in venous noradrenaline during the 20‐year period was actually related to the difference in BP between screening and inclusion time for those who converted from normotension to hypertension during that follow‐up. Arterial noradrenaline at baseline was negatively correlated to BP at the time of follow‐up in the subjects who remained normotensive, and was the only independent predictor of BP 20 years later in that group. These results should be interpreted with caution, as it is a small group of subjects; however, one might speculate that this association could reflect healthy physiological BP regulation mechanisms in those men that remained normotensive, namely that BP elevation led to a suppression of SNS, and thereby lower noradrenaline values. This negative correlation may therefore actually support the notion of increased sympathetic tone in hypertension Citation[32], and that the subjects with sustained normotension may have an inherent protection against sympathetic overactivity. Furthermore, we did not observe such a negative relationship between noradrenaline and BP in those who were categorized as hypertensive in 1984 or those who converted from normotension to hypertension during the follow‐up. There seemed to be a positive relationship between the change in venous plasma noradrenaline over the 20‐year period and the rise in MBP between screening and investigation in 1984. This was apparent for the whole group, but the relationship persisted only in the NTHT group and not in the NTNT group. One could argue that the putative inherent protection against sympathetic overactivity in the NTNT can be extended to sympathoadrenal influence, i.e. the lack of prediction of arterial plasma adrenaline regarding the development of BP in that group.
In this present follow‐up study of 60‐year‐old men recruited from The Oslo Study 20 years ago, we were able to re‐examine 77.3% of the originally normotensive men and 62.8% of the originally hypertensive men. BP at baseline did not differ between those who participated in the follow‐up study and those who did not. It would therefore be fair to assume that selection errors to a large extent were avoided even though a larger participation of the originally hypertensive group would have been preferable. The power of the study is limited by the relatively few participants at both baseline and follow‐up. The strength of the study is the longitudinal study protocol with a follow‐up period of 20 years in addition to the unique homogeneity of the group and the number of parameters, including arterial catecholamines, from 20 years ago that can be related to later BP development. Furthermore, the fact that we used exactly the same method to analyse plasma catecholamines now and 20 years ago increases the value of our study. Our group has previously published that measurements of arterial plasma catecholamines seem to be a more sensitive tool for detecting hypertensive–normotensive differences than venous plasma catecholamines Citation[5], Citation[33]. Arterial measurements would therefore have been of interest to have at follow‐up but were not included in the protocol for ethical reasons, i.e. arterial sampling using the same method as in 1984 with indwelling catheter now being considered to be a procedure too invasive to be acceptable.
Screening BP at baseline, albeit well within the normotensive range, could identify participants that developed hypertension 20 years later and it was the only independent predictor of BP development in the group as a whole. The reduction of BP from a stressful screening to the time of investigation under standardized condition and rest actually was reflected in the increase in BP during the next 20 years in this group. Arterial adrenaline at baseline was found to be an independent predictor of BP at follow‐up in the men who converted from normotension to hypertension, suggesting the importance of sympathoadrenal activity for the development of hypertension. Although there was a difference in arterial plasma noradrenaline in the hypertensives and normotensives at baseline, this difference did not reach statistical significance for venous noradrenaline 20 years later, although numerically the difference was of similar magnitude. Furthermore, in our 20‐year follow‐up study, we confirmed previous observations from cross‐sectional studies that plasma noradrenaline increased with age while plasma adrenaline did not change.
Our study demonstrates the importance of longitudinal observations and it lends support to a role of the sympathoadrenal system in development and maintenance of hypertension.
Acknowledgements
The authors wish to thank the technical staff at the Cardiovascular and Renal research laboratorium at Ulleval University hospital for expert technical assistance. This work was supported by the Norwegian Renal Association.
References
- Oparil S., Zaman M. A., Calhoun D. A. Pathogenesis of hypertension. Ann Intern Med 2003; 139: 761–776
- Julius S., Majahalme S. The changing face of sympathetic overactivity in hypertension. Ann Med 2000; 32: 365–370
- Kjeldsen S. E., Flaaten B., Eide I., Helgeland A., Leren P. Increased peripheral release of noradrenaline and uptake of adrenaline in essential hypertension?. Clin Sci (Lond) 1981; 61(Suppl 7)215s–217s
- Kjeldsen S. E., Gjesdal K., Eide I., Aakesson I., Amundsen R., Foss O. P., et al. Increased beta‐thromboglobulin in essential hypertension: Interactions between arterial plasma adrenaline, platelet function and blood lipids. Acta Med Scand 1983; 213: 369–373
- Kjeldsen S. E., Schork N. J., Leren P., Eide I. K. Arterial plasma norepinephrine correlates to blood pressure in middle‐aged men with sustained essential hypertension. Am Heart J 1989; 118: 775–781
- Reims H. M., Sevre K., Fossum E., Hoieggen A., Eide I., Kjeldsen S. E. Plasma catecholamines, blood pressure responses and perceived stress during mental arithmetic stress in young men. Blood Press 2004; 13: 287–294
- Rostrup M., Westheim A., Kjeldsen S. E., Eide I. Cardiovascular reactivity, coronary risk factors, and sympathetic activity in young men. Hypertension 1993; 22: 891–899
- Leren P., Askevold E. M., Foss O. P., Froili A., Grymyr D., Helgeland A., et al. The Oslo study. Cardiovascular disease in middle‐aged and young Oslo men. Acta Med Scand 1975; 588: 1–38
- Hoieggen A., Fossum E., Moan A., Rostrup M., Eide I. K., Kjeldsen S. E. Effects of hyperinsulinemia on sympathetic responses to mental stress. Am J Hypertens 2000; 13((1 Pt 1))21–28
- Os I., Kjeldsen S. E., Aakesson I., Skjoto J., Eide I., Hjermann I., et al. Evidence of age‐related variation in plasma vasopressin of normotensive men. Scand J Clin Lab Invest 1985; 45: 263–268
- Os I., Kjeldsen S. E., Westheim A., Lande K., Norman N., Hjermann I., et al. Endocrine and haemodynamic responses to graded dopamine infusion in essential hypertension. Scand J Clin Lab Invest 1987; 47: 371–377
- Os I., Kjeldsen S. E., Westheim A., Aakesson I., Norman N., Enger E., et al. Decreased central dopaminergic activity in essential hypertension. J Hypertens 1987; 5: 191–197
- Planz G., Wiethold G., Appel E., Bohmer D., Palm D., Grobecker H. Correlation between increased dopamine‐beta‐hydroxylase activity and catecholamine concentration in plasma: Determination of acute changes in sympathetic activity in man. Eur J Clin Pharmacol 1975; 8: 181–188
- Os I., Kjeldsen S. E., Westheim A., Akesson I., Eide I., Skjoto J., et al. Aging and urinary vasopressin excretion in healthy men. Scand J Urol Nephrol 1987; 21: 235–239
- Goldstein D. S. Plasma catecholamines and essential hypertension. An analytical review. Hypertension 1983; 5: 86–99
- Anderson E. A., Sinkey C. A., Lawton W. J., Mark A. L. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension 1989; 14: 177–183
- Mancia G. Bjorn Folkow Award Lecture. The sympathetic nervous system in hypertension. J Hypertens 1997; 15((12 Pt 2))1553–1565
- Grassi G., Cattaneo B. M., Seravalle G., Lanfranchi A., Mancia G. Baroreflex control of sympathetic nerve activity in essential and secondary hypertension. Hypertension 1998; 31: 68–72
- Narkiewicz K., Phillips B. G., Kato M., Hering D., Bieniaszewski L., Somers V. K. Gender‐selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension 2005; 45: 522–525
- Ng A. V., Callister R., Johnson D. G., Seals D. R. Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension 1993; 21: 498–503
- 2003 European Society of Hypertension‐European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 21. 1011–1053
- O'Brien E., Asmar R., Beilin L., Imai Y., Mallion J. M., Mancia G., et al. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens 2003; 21: 821–848
- Reims H., Fossum E., Kjeldsen S. E., Julius S. Home blood pressure monitoring. Current knowledge and directions for future research. Blood Press 2001; 10: 271–287
- Peuler J. D., Johnson G. A. Simultaneous single isotope radioenzymatic assay of plasma norepinephrine, epinephrine and dopamine. Life Sci 1977; 21: 625–636
- Hjemdahl P. Inter‐laboratory comparison of plasma catecholamine determinations using several different assays. Acta Physiol Scand 1984; 527: 43–54
- Fossum E., Hoieggen A., Reims H. M., Moan A., Rostrup M., Eide I., et al. High screening blood pressure is related to sympathetic nervous system activity and insulin resistance in healthy young men. Blood Press 2004; 13: 89–94
- Masuo K., Mikami H., Ogihara T., Tuck M. L. Sympathetic nerve hyperactivity precedes hyperinsulinemia and blood pressure elevation in a young, nonobese Japanese population. Am J Hypertens 1997; 10: 77–83
- Masuo K., Kawaguchi H., Mikami H., Ogihara T., Tuck M. L. Serum uric acid and plasma norepinephrine concentrations predict subsequent weight gain and blood pressure elevation. Hypertension 2003; 42: 474–480
- Goldstein D. S., Lake C. R., Chernow B., Ziegler M. G., Coleman M. D., Taylor A. A., et al. Age‐dependence of hypertensive‐normotensive differences in plasma norepinephrine. Hypertension 1983; 5: 100–104
- Seals D. R., Esler M. D. Human ageing and the sympathoadrenal system. J Physiol 2000; 528((Pt 3))407–417
- Ziegler M. G., Lake C. R., Kopin I. J. Plasma noradrenaline increases with age. Nature 1976; 261: 333–335
- Stamler J., Stamler R., Rhomberg P., Dyer A., Berkson D. M., Reedus W., et al. Multivariate analysis of the relationship of six variables to blood pressure: Findings from Chicago community surveys, 1965–1971. J Chronic Dis 1975; 28: 499–525
- Kjeldsen S. E., Flaaten B., Eide I., Helgeland A., Leren P. Evidence of increased peripheral catecholamine release in patients with long‐standing, untreated essential hypertension. Scand J Clin Lab Invest 1982; 42: 217–223