Abstract
Aims. This study sought to compare the antihypertensive efficacy and tolerability of a fixed‐dose combination with amlodipine/benazepril with that of amlodipine monotherapy in Chinese hypertensive subjects. Results. This multicenter, double‐blind, 8‐week study randomized 111 patients to fixed‐dose amlodipine besylate/benazepril HCl (2.5/5 mg/day titrated to 5/10 mg/day as needed at week 4 to reach goal blood pressure (BP) <140/90 mmHg) or amlodipine besylate monotherapy (5 mg/day titrated to 10 mg/day as needed). At week 8, patients randomized to combination therapy compared with monotherapy had a comparable BP control rate (56.0% vs 46.2%; p = 0.32). Fixed‐dose combination resulted in similar reductions in sitting systolic (SBP) and diastolic BP (DBP) compared with monotherapy (SBP: −19.3±12.5 vs −20.9±13.3 mmHg; DBP: −9.2±10.4 vs −11.3±9.3 mmHg; both p = NS). Safety profiles did not differ between groups, but cough was more common in the combination group (11.0% vs 0%; p = 0.013). Conclusions. In this group of patients, comparable antihypertensive effects were seen with the fixed‐dose combination therapy, compared with amlodipine monotherapy. Both treatments appeared well tolerated in the studied population, but cough was more common in the fixed‐dose combination group.
Introduction
Uncontrolled hypertension is a well‐established major risk for cardiovascular events, including stroke, coronary artery disease and heart failure Citation[1,2]. The risk of these events decreases directly with the magnitude of sustained reduction in blood pressure (BP) Citation[3]. To benefit maximally from antihypertensive treatment BP should be brought to below 140/90 mmHg in every hypertensive patient, and even lower (<130/80 mmHg) in the presence of diabetes or renal dysfunction, or in those patients with a history of stroke or myocardial infarction Citation[4]. Such targets cannot usually be reached using monotherapies. The frequent need to treat hypertensive patients with more than one agent in order to reach target BP is highlighted in recent antihypertensive treatment guidelines Citation[4–6]. There is abundant randomized, controlled trial data Citation[7] that low‐dose drug combination regimens increase efficacy and reduce adverse effects. In recognition of the superiority of combination therapy, the 2007 European Guidelines Citation[4] suggested beginning combination therapy with two drugs at low doses, regardless of the degree of hypertension. On the other hand, the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) Citation[6] considered only specific patients with more severe hypertension [stage 2 and above, i.e. systolic BP (SBP) >160 mmHg and diastolic (DBP) >100 mmHg] to be candidates for combined therapies as first‐line treatment.
Rational drug combinations include angiotensin‐converting enzyme (ACE) inhibitors with long‐acting dihydropyridine calcium‐channel blockers (CCB), since co‐administration of the ACE inhibitors and CCB has been reported to have enhanced BP‐lowering efficacy with less dose‐dependent adverse effects Citation[8–15]. In particular, the Anglo‐Scandinavian Cardiac Outcomes Trial–Blood Pressure Lowering Arm (ASCOT‐BPLA) study showed that an antihypertensive strategy based on amlodipine, with perindopril added as required, could significantly reduce total cardiovascular events and strokes, compared with an atenolol‐based strategy, with the diuretic bendroflumethiazide added as required, in hypertensive patients with additional cardiovascular risk factors Citation[16]. This study strengthens the rationale to use the combination of an ACE inhibitor and a CCB in the treatment of hypertension.
The fixed‐dose combination described here used the ACE inhibitor benazepril hydrochloride (Bez) and the long‐acting CCB amlodipine besylate (Amd). Bez is a nonsulfhydryl, selective inhibitor of ACE, which has been reported to be effective and well tolerated in the treatment of hypertension at a dosage of 10–40 mg once daily Citation[17]. Amd is a long‐acting dihydropyridine derivative that has been shown to exert antihypertensive and antianginal effects with once‐daily doses of 5–10 mg Citation[18].
Not much is known about the efficacy and tolerability of a fixed Amd/Bez combination at low doses as a first‐line treatment of mild to moderate hypertension, particularly in Chinese populations. Therefore, this study evaluated the antihypertensive efficacy and tolerability of the fixed‐dose Amd/Bez combination in comparison with Amd alone in patients with mild to moderate essential hypertension.
Patients and methods
Patient population
Chinese men and women between 20 and 75 years of age with uncomplicated essential hypertension were eligible for inclusion in this trial if they were either with sitting DBP ⩾90 and <110 mmHg or with SBP ⩾140 and <180 mmHg between at screening, run‐in and randomization visits. Patients with any of the following were excluded: secondary hypertension; history of acute coronary syndrome; decompensated congestive heart failure; hypertensive encephalopathy; cerebrovascular accident; hypertensive retinopathy grade 3 or 4; sick sinus syndrome; second or third degree atrioventricular block; significant hepatic (aspartate or alanine aminotransferase >3 times the upper limit of normal), renal (creatinine >1.5 times the upper limit of normal), metabolic (including uncontrolled diabetes mellitus with HbA1c>10 %), hematological, immunological or respiratory disorders; gastrointestinal tract diseases; known hypersensitivity to study drugs; or a history of alcohol or drug abuse. Women of childbearing potential not using an effective method of contraception and pregnant and breast‐feeding women were also excluded.
Study design
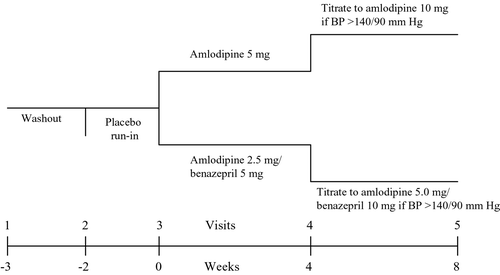
This was a randomized, double‐blind, 8‐week, active‐controlled, parallel‐group study that was conducted in three medical centers across Taiwan from February 2004 through December 2005. The protocol was approved by the ethics committee of each participating center and prior written informed consent was obtained from all patients. The study design is shown in . There were five study visits: at screening (visit 1), at the beginning of the placebo run‐in period (visit 2), at randomization (visit 3) and after 4 weeks (visit 4), and 8 weeks of active treatment (visit 5).
At the initial screening visit, patients underwent a complete physical examination and laboratory assessment. Following the screening visit, eligible subjects entered into a 2‐week, single‐blind, placebo run‐in period. Patients who were receiving antihypertensive treatment at the screening visit first underwent a tapering‐off period of at least 1 week before enrolling in the placebo run‐in period. After the run‐in period, all patients who fulfilled the entry criteria were allocated randomly in 1:1 ratio to receive Amd 2.5 mg/Bez 5 mg or Amd 5 mg OD for 4 weeks. A computer‐generated randomization schedule was used to guarantee that patients were distributed equally among the two treatment groups and within each center. After 4 weeks of treatment, responders whose sitting DBP<90 mmHg and SBP<140 mmHg continued the same dose regimen through the end of the study. If the BP did not achieve treatment goal (SBP⩾140 mmHg or DBP⩾90 mmHg) at the end of 4 weeks of treatment, the dose of either regimen was doubled to Amd 5 mg/Bez 10 mg or Amd 10 mg OD for the following 4 weeks.
Study drugs were taken immediately before breakfast throughout the study period of 8 weeks. Other antihypertensive drugs and any other drugs that might affect BP were prohibited during the trial period. At each visit, BP was measured using a calibrated mercury sphygmomanometer with cuff of an appropriate size at 24 h (range 22–26 h) after the last dose of study medication, that is, at trough of drug effect. Phase I and V (disappearance) Korotkoff sounds were used to identify SBP and DBP, respectively. Every attempt was made to see that BP of each patient was recorded at the same arm, the same time of day by the same individual using the same equipment. The clinic BP reference value for each patient was the average of three serial measurements taken at 3‐min intervals after the patient had rested for 10 min in the sitting position. Heart rate was determined by cardiac auscultation for at least 30 s in the sitting position immediately after the first BP measurement. Compliance was assessed by counting unused tablets returned at scheduled visits, and counseling was provided on an individual basis as required.
At visits 1, 4 and 5, laboratory tests were performed. The laboratory examinations consisted of hematology (i.e. full cell count, hemoglobin and hematocrit) and blood biochemistry (i.e. glucose, blood urea nitrogen, uric acid, total cholesterol, alkaline phosphatase, aspartate transaminase, alanine transaminase, creatinine, potassium and total cholesterol).
An adverse event (AE) was defined as any unfavorable symptom, sign or condition that occurred after the initiation of treatment, regardless of its relation to study drug. At each visit, AEs, including ankle edema, could be reported by the patient, elicited by general questioning, detected on physical examination, in a laboratory test, or by other means. The incidence of AEs was tabulated by treatment group, according to severity and to relationship to study drug.
Statistical analyses
A sample size of 50 in each group had an 80% power to detect a mean difference of 3.5 mmHg in trough DBP assuming that the standard deviation (SD) was 7 mmHg, using a two‐group t‐test with a 0.05 two‐sided significance level. A total of 100 patients were calculated to afford 80% power to show that combination therapy is at least as effective as monotherapy with respect to the mean reduction in trough sitting DBP at week 8.
Descriptive statistics were presented for variables of continuous type and frequency tables were provided for categorical data. The two treatment groups were compared for demographics characteristics, efficacy and safety by two‐way analysis of covariance (ANCOVA). All efficacy analyses were performed on an intent‐to‐treat (ITT) basis, defined as subjects who received at least one dose of a randomized study drug and had at least one post‐baseline BP measurement. Efficacy evaluations were performed at the evaluation visit and the final visit. The primary efficacy endpoint was the control rate, which was defined as the proportion of subjects with a final sitting SBP<140 mmHg and DBP<90 mmHg at week 8. The secondary efficacy endpoints consisted of the net changes of the mean sitting SBP, DBP and the heart rate from baseline to endpoint. ANCOVA was used to incorporate the baseline BP as the covariate in the model, and the difference in BP as the dependent variable. The 95% confidence intervals (CIs) for the differences between treatments were calculated. The safety analysis was based on all randomized patients who received at least one dose of study medication during the double‐blind treatment phase. A p‐value <0.05 was deemed statistically significant.
Results
Patients
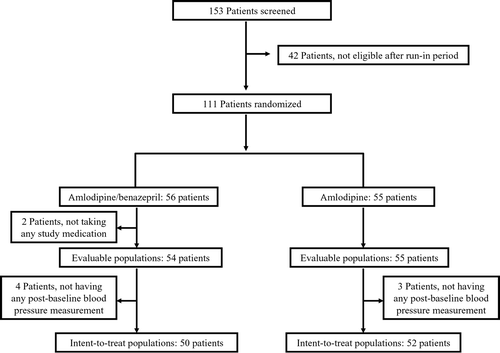
Of 153 patients screened, 42 who failed to meet the randomization criteria were excluded at the end of the run‐in period. There were 111 patients were randomized to treatment, 56 receiving combination therapy and 55 receiving monotherapy, respectively. Disposition of patients and study populations are shown in . Of the randomized subjects, nine (six in the combination group and three in monotherapy group) were dropped from ITT analysis due to not take any study medication (n = 2) or not having at least one post‐treatment BP measurement (n = 7) during the double‐blind treatment phase. Thus, data from 102 patients (50 in the combination group and 52 in the monotherapy group) were used for the ITT analysis. The baseline demographic characteristics and vital signs of the ITT population are presented in . There were no significant differences between study groups for age, sex, body weight, height, baseline sitting SBP, DBP, heart rate, serum creatinine, the proportion of patients with isolated systolic hypertension or prior antihypertensive medications. At randomization (visit 3), mean (SD) sitting SBP and DBP were 152.0 (12.7) mmHg and 95.5 (9.8) mmHg, respectively, in the combination group and 153.6 (13.3) mmHg and 96.5 (10.0) mmHg, respectively, in the monotherapy group. The mean (SD) compliance rates were 88.6% (19.8%) and 91.4% (18.7%) in the combination and monotherapy groups, respectively, indicating good treatment compliance in either treatment arm.
Table I. Baseline demographic and clinical characteristics of study patients (intent‐to treat populations).
Primary efficacy outcome
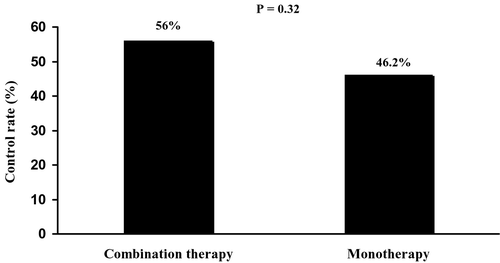
There was no significant difference in the proportion of patients receiving a double dose of medication after the initial 4 weeks treatment, with 36.5% of those in the combination group (19/50) and 32.7 % of those in the monotherapy group (17/52). The control rate in each treatment group who attained BP goals <140/90 mmHg from baseline to the end of study was evaluated. The primary endpoint assessment using ITT analysis showed that similar BP control rates were observed with combination therapy (56.0%) when compared with monotherapy (46.2%) (p = 0.32, ).
Secondary efficacy outcomes
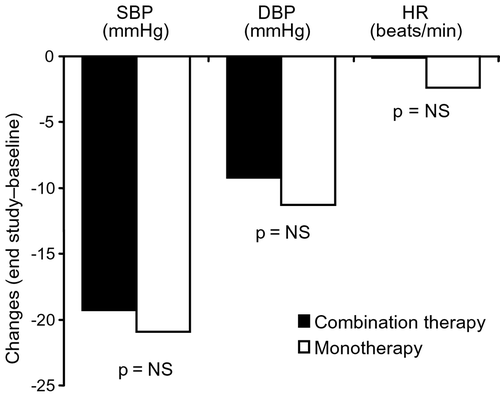
For the secondary efficacy outcome of change in mean sitting SBP and DBP from baseline to end of the study, the mean (SD) reductions in sitting SBP/DBP for fixed‐dose combination versus monotherapy were −19.3 (12.5) mmHg [95% CI −16.4 to −22.3]/−9.2 (10.4) mmHg [95% CI −6.9 to −11.7] and −20.9 (13.3) mmHg [95% CI −17.7 to −23.4]/−11.3 (9.3) mmHg [95% CI −8.7 to −13.3], respectively (all p>0.05) (). No statistically significant differences were also observed among the treatment groups with respect to net change of heart rate in a sitting position. The mean (SD) heart rates in the combination group were 74.5 (8.7) and 74.4 (8.9) beats/min at baseline and final visit, respectively. In the monotherapy group, the mean (SD) heart rates were 76.4 (8.0) and 74.0 (11.0) beats/min at baseline and final visit, respectively.
Safety profiles
The safety population included all 109 patients (54 in the combination therapy and 55 in the monotherapy) who received at least one dose of study medication. There were 66 treatment‐emergent AEs (TEAEs; drug‐related or not), 33 in each treatment groups reported on 25 patients (46.3%) receiving fixed‐dose combination and 22 (40.0%) receiving monotherapy at endpoint. The majority of TEAEs reported were mild to moderate in severity. Drug‐related AEs from the study were significantly frequent in the fixed‐dose combination group than in the monotherapy group (13.0% vs 0%, p = 0.006), with none of these events to be classified as a serious AE. The higher incidence of drug‐related AEs in the combination group was due to the significantly higher incidence of cough, compared with monotherapy group. In contrast, AEs causing discontinuation from the study were not statistically significant between groups (combination vs monotherapy: 0% vs 5.4%, p = NS). Ankle edema was complained of or was clinically evident in two subjects with combination therapy and in one with monotherapy during the active treatment period (p = NS). The most frequently TEAEs, reported from all subjects in the safety population at an incidence of >1% for either treatment group, were listed in . Cough was more commonly reported with combination therapy than with monotherapy (11.0% vs 0%; p = 0.013). The incidence of pedal edema was low in both groups, reported in two (3.7%) patients in the combination group and in one (1.8%) in the monotherapy group. Dizziness was reported at an incidence of 3.7% in the combination group and 5.5% in the monotherapy group. None of the clinical laboratory parameters was modified by trial drugs.
Table II. Adverse events (AEs) observed during active treatment.
Discussion
Fixed‐dose combinations typically were reported to offer the advantages of greater BP reductions, and reduced the incidence of dose‐dependent adverse effects. In the present study, however, fixed‐dose combination produced a similar BP control rate as monotherapy after 8 weeks of active treatment. The reductions in SBP and DBP were also similar in each treatment regimen. Both treatments appeared well tolerated in the studied population, but with significantly more drug‐related AEs, mostly cough, in patients receiving fixed‐dose combination. The incidence of pedal edema was similarly low in both groups. The results of this study did not support the recommendation in the 2007 European practice guideline for the initial use of combination therapy at low doses in patients with essential hypertension, in that the use of initial fixed‐dose combination did not result in a greater proportion of patients achieving target BP goals or in a more SBP/DBP reductions, along with similar tolerability but with more drug‐related AE‐cough.
As a combination product, fixed‐dose Amd/Bez combinations have been extensively studied to demonstrate clinical efficacy at a variety of larger doses of 2.5/10, 5/10, 5/20, 5/40, 10/20 and 10/40 mg/day in hypertensive populations Citation[8–15]. In the Systolic Evaluation of Lotrel Efficacy and Comparative Therapies (SELECT) study, such combination was reported to exert a greater BP‐reducing efficacy than either component as monotherapy Citation[15]. In addition, fixed‐dose combination with Amd/Bez (5/20 mg/day titrated to 10/20 mg/day) resulted in significantly greater BP reductions and attainment of BP goals compared with Amd monotherapy (5 mg/day titrated to 10 mg/day) in patients with stage 2 hypertension Citation[14]. In contrast, the doses described here in Amd/Bez combination were low (2.5/5 mg titrated to 5/10 mg), which might in part account for the unsatisfied result that the fixed‐dose combination did not attain significantly higher BP control rate or BP reductions than monotherapy did. Of note, most of the studied patients in these previous studies are white or black. To the best of our knowledge, the present study is the first study to report the results of fixed‐dose Amd/Bez combination in Chinese subjects with essential hypertension. Indeed, there are patterns of genetic variations (e.g. CLCNKA‐B, BSND, NEDD4L) between populations, which may possibly explain such results Citation[19]. There may also be other common genetic variants that could possibly explain the discrepancy usually seen in Caucasian populations as compared with the present results in a Chinese population.
The overall safety profiles of the two treatments did not differ significantly, but with more cough in the combination group than in the monotherapy group. Indeed, dry cough, a common side‐effect of ACE inhibitors, was more often seen in Chinese patients receiving combination therapy than in those receiving CCB monotherapy. The relatively high incidence of cough in the patients receiving combination therapy reflects the bothersome adverse reaction of ACE inhibitors in Chinese people Citation[20,21]. One expected drawback of starting treatment with fixed‐dose combination is that with the introduction of another molecule into the therapeutic regimen, the hazard of dose‐independent side‐effects will increase.
The incidence of pedal edema was low in both groups (fixed‐dose combination vs monotherapy; 3.7% vs 1.8%, respectively, p = NS). Edema formation is a frequent side‐effect during administration of dihydropyridine CCB with this AE reportedly occurring in up to 33.3% of patients administered with Amd Citation[22,23]. Data from previous studies have been reported that nearly one‐fourth of patients with inadequate BP control on low‐dose Amd experience peripheral edema when the dose of Amd is doubled from 5 to 10 mg Citation[10],Citation[24]. Another large practice‐based clinical trial assessed the tolerability of Amd/Bez combination in 7912 hypertensive subjects who were being treated with Amd monotherapy Citation[25]. For subjects who had experienced peripheral edema while on monotherapy, 85% experienced improvement after switching to Amd/Bez combination therapy. In the present study, it was unusual for the either group to have extremely low incidences of pedal edema. This was probably due to the relatively small number of patients in this study, as well as the short duration of the follow‐up period. Indeed, there is some data supporting a lower incidence of edema in Asian populations Citation[26].
There is no doubt that effective BP control can be achieved in the majority of hypertensive patients by pharmacological treatment. However, the choice of the initial medication is important, as both its efficacy and tolerability might influence daily and long‐term adherence. Finally, it should be stressed that the present observations are not necessarily generalized to all fixed‐dose combinations or monotherapies, since the studied results of efficacy and tolerability may depend on the choice of individual agents, dosing regimens and racial difference selected for treatment.
Conclusions
In this group of Chinese patients with mild to moderate hypertension, comparable BP lowering effects and control rate were seen with the fixed‐dose Amd/Bez combination (2.5/5 mg/day titrated to 5/10 mg/day), compared with Amd monotherapy (5 mg/day titrated to 10 mg/day) after 8 weeks of active treatment. Both treatments appeared well tolerated in the studied population, but with more cough in patients receiving fixed‐dose combination.
Acknowledgments
This study was sponsored by the TTY Biopharm Company Limited, Taipei, Taiwan. The authors have no conflicts of interest directly relevant to the contents of this article.
References
- MacMahon S., Peto R., Cutler J., Collins R., Sorlie P., Neaton J., et al. Blood pressure, stroke, and coronary heart disease: Prolonged differences in blood pressure. Lancet 1990; 335: 765–774
- Stamler J., Stamler R., Neaton J. D. Blood pressure, systolic and diastolic, and cardiovascular risks: US population data. Arch Intern Med 1993; 153: 598–615
- Collins R., Peto R., MacMahon S., Hebert P., Fiebach N. H., Eberlein K. A., et al. Blood pressure, stroke, and coronary heart disease. Part 2, Short‐term reductions in blood pressure: Overview of randomized drug trials in their epidemiological context. Lancet 1990; 335: 827–838
- Mancia G., De Backer G., Dominiczak A., Cifkova R., Fagard R., Germano G., et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2007; 25: 1105–1187
- World Health Organization International Society of Hypertension Writing Group. World Health Organization (WHO)/ International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens 2003; 21: 1983–1992
- Chobanian A. V., Bakris G. L., Black H. R., Cushman W. C., Green L. A., Izzo J. L., Jr., et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. JAMA 2003; 289: 2560–2572
- Law M. R., Wald N. J., Morris J. K., Jordan R. E. Value of low dose combination treatment with blood pressure lowering drugs: Analysis of 354 randomised trials. BMJ 2003; 326: 1427–1435
- Frishman W. H., RAM C. V., McMahon F. G., Chrysant S. G., Graff A., Kupiec J. W., et al. Comparison of amlodipine and benazepril monotherapy to amlodipine plus benazepril in patients with systemic hypertension: A randomized, double‐blind, placebo‐controlled, parallel‐group study. The Benazepril/Amlodipine Study Group. J Clin Pharmacol 1995; 35: 1060–1066
- Kuschnir E., Acuña E., Sevilla D., Vasquez J., Bendersky M., Resk J., et al. Treatment of patients with essential hypertension: Amlodipine 5 mg/benazepril 20 mg compared with amlodipine 5 mg, benazepril 20 mg, and placebo. Clin Ther 1996; 18: 1213–1224
- Messerli F. H., Oparil S., Feng Z. Comparison of efficacy and side effects of combination therapy of angiotensin‐converting enzyme inhibitor (benazepril) with calcium antagonist (either nifedipine or amlodipine) versus high‐dose calcium antagonist monotherapy for systemic hypertension. Am J Cardiol 2000; 86: 1182–1187
- Pool J., Kaihlanen P., Lewis G., Ginsberg D., Oparil S., Glazer R., et al. Once‐daily treatment of patients with hypertension: A placebo‐controlled study of amlodipine and benazepril vs amlodipine or benazepril alone. J Hum Hypertens 2001; 15: 495–498
- Messerli F. H., Weir M. R., Neutel J. M. Combination therapy of amlodipine/benazepril versus monotherapy of amlodipine in a practice‐based setting. Am J Hypertens 2002; 15: 550–556
- Chrysant S. G., Bakris G. L. Amlodipine/benazepril combination therapy for hypertensive patients nonresponsive to benazepril monotherapy. Am J Hypertens 2004; 17: 590–596
- Jamerson K. A., Nwose O., Jean‐Louis L., Schofield L., Purkayastha D., Baron M. Initial angiotensin‐converting enzyme inhibitor/calcium channel blocker combination therapy achieves superior blood pressure control compared with calcium channel blocker monotherapy in patients with stage 2 hypertension. Am J Hypertens 2004; 17: 495–501
- Neutel J. M., Smith D. H., Weber M. A., Schofield L., Purkayastha D., Gatlin M. Efficacy of combination therapy for systolic blood pressure in patients with severe systolic hypertension: The Systolic Evaluation of Lotrel Efficacy and Comparative Therapies (SELECT) study. J Clin Hypertens 2005; 7: 641–646
- Dahlof B., Sever P. S., Poulter N. R., Wedel H., Beevers D. G., Caulfield M., et al. ASCOT Investigators. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo‐Scandinavian Cardiac Outcomes Trial‐Blood Pressure Lowering Arm (ASCOT‐BPLA): A multicentre randomised controlled trial. Lancet 2005; 366: 895–906
- Balfour J. A., Goa K. L. Benazepril: A review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in hypertension and congestive heart failure. Drugs 1991; 42: 511–539
- Murdoch D., Heel R. C. Amlodipine: A review of its pharmacodynamic properties and therapeutic use in cardiovascular disease. Drugs 1991; 41: 478–505
- Sile S., Velez D. R., Gillani N. B., Alexander C. A., George A. L., Jr., Williams S. M. Haplotype diversity in four genes (CLCNKA, CLCNKB, BSND, NEDD4L) involved in renal salt reabsorption. Hum Hered 2008; 65: 33–46
- Chan P., Lin C. N., Tomlinson B., Lin T. H., Lee Y. S. Additive effects of diltiazem and lisinopril in the treatment of elderly patients with mild‐to‐moderate hypertension. Am J Hypertens 1997; 10: 743–749
- Lee Y. J., Tsai J. C. Angiotensin‐converting enzyme gene insertion/deletion, not bradykinin B2 receptor‐58T/C gene polymorphism, associated with angiotensin‐converting enzyme inhibitor‐related cough in Chinese female patients with non‐insulin‐dependent diabetes mellitus. Metabolism 2001; 50: 1346–1350
- Pedrinelli R., Dell'Omo G., Melillo E., Mariani M. Amlodipine, enalapril, and dependent leg edema in essential hypertension. Hypertension 2000; 35: 621–625
- Lund‐Johansen P., Stranden E., Helberg S., Wessel‐Aas T., Risberg K., Rønnevik P. K., et al. Quantification of leg oedema in postmenopausal hypertensive patients treated with lercanidipine or amlodipine. J Hypertens 2003; 21: 1003–1010
- Kloner R. A., Sowers J. R., DiBona G. F., Gaffney M., Wein M. Sex‐ and age‐related antihypertensive effects of amlodipine. The Amlodipine Cardiovascular Community Trial Study Group. Am J Cardiol 1996; 713–722, 77
- Taylor A. A., Shoheiber O. Adherence to antihypertensive therapy with fixed‐dose amlodipine besylate/benazepril HCl versus comparable component‐based therapy. Congest Heart Fail 2003; 9: 324–332
- Park S., Chung N., Kwon J., Yoon J. H., Kim Y. J., Han D. S., et al. Results of a multicenter, 8‐week, parallel‐group, randomized, double‐blind, double‐dummy, Phase III clinical trial to evaluate the efficacy and tolerability of amlodipine maleate versus amlodipine besylate in Korean patients with mild to moderate hypertension. Clin Ther 2005; 27: 441–450