Abstract
Objective. To investigate whether the yield of population-based diabetes screening is influenced by characteristics of the general practitioner (GP) and the practice. Design. Cross-sectional study. Setting. Seventy-nine general practices in the south-western region of the Netherlands. Subjects. From 2002 to 2004, 56 978 people were screened for diabetes. GPs completed a questionnaire containing items on the GP (age, gender, employment, special interest in diabetes, providing insulin therapy) and the practice (setting, location, number of patients from ethnic minority groups, specific diabetes clinic, involvement of practice assistant, practice nurse or diabetes nurse in diabetes care). Main outcome measures. The ratio screen-detected diabetic patients/known diabetic patients per practice (SDM/KDM) and the number of detected diabetic patients per practice adjusted for practice size and age distribution (SDM per standardized practice). Results. The yield of screening per practice varied widely. Higher age of the GP (regression coefficient 0.20; 95% confidence interval, CI 0.07–0.34), urban location (−4.60; 95% CI −6.41 to −2.78) and involvement of the practice assistant (2.27; 95% CI 0.49–4.06) were independently associated with SDM/KDM. Using the other outcome variable, results were similar. Additionally, cooperation with a diabetes nurse was associated with a lower yield. Conclusion. A lower yield of screening, reflecting a lower prevalence of undiagnosed diabetes, was found in practices of younger GPs and in urban practices. A lower yield was not associated with an appropriate practice organization regarding diabetes care nor with a specialty of the GP in diabetes. The wide variation in the yield of screening stresses the importance of a screening programme in each general practice.
Introduction
Early diagnosis of diabetes might be beneficial, although definitive studies of the effectiveness of screening for type 2 diabetes are not available Citation[1]. Screening for diabetes can be distinguished in population-based screening and targeted screening directed at high-risk individuals. Both strategies are often combined. Opportunistic screening or case-finding involves screening during routine encounters with the health care system. The American Diabetes Association stated that there is sufficient indirect evidence to justify opportunistic screening for diabetes in a clinical setting Citation[2].
It is unknown whether the yield of diabetes screening in primary care is influenced by practice and GP characteristics.
A lower yield, reflecting a lower prevalence of undiagnosed diabetes, was found in practices of younger GPs and in urban practices.
A lower yield of screening, was not associated with an appropriate practice organization regarding diabetes care.
The yield in the practices varied widely, underlining the importance of a diabetes screening programme in each practice.
The yields of screening programmes vary depending on used algorithms and population characteristics. Generally, the yields of diabetes screening programmes are low Citation[3]. In the Netherlands, the Dutch College of General Practitioners recommends case-finding in general practice in several well-defined categories of patients at high-risk of having undiagnosed diabetes Citation[4]. Such case-finding may be more cost-effective and more pragmatic than population-based screening Citation[5], Citation[6].
In order to optimize detecting undiagnosed diabetes in general practice, it is relevant to know whether it is possible to identify general practitioners (GPs) and practices with larger numbers of unidentified diabetic patients. Previous studies have demonstrated that quality improvement programmes regarding both practice organizational factors and features of the GPs improve the provision of diabetes care Citation[7–9]. In addition, it has been shown that if a GP has a specialty in diabetes, a better quality of diabetes care was ensured Citation[10]. However, improvements occurred primarily in the process outcomes, rather than in the patient outcomes. Hansen et al. found that characteristics of GPs, such as interest in diabetes, experience, practice type and weekly working hours, did not predict their patients’ glycaemic control Citation[11]. To our knowledge, it has not been investigated whether an appropriate practice organization and special interest or skills of GPs regarding diabetes care are associated with fewer undiagnosed diabetic patients in the practice and, consequently, a lower yield of screening.
This study aims to investigate whether and to what extent the yield of population-based diabetes screening in primary care is influenced by GP and practice characteristics.
Material and methods
This study was conducted within the framework of the ADDITION study (Anglo-Danish-Dutch Study of Intensive Treatment in People with Screen-Detected Diabetes in Primary Care). ADDITION is a randomized trial of a target-driven approach to intensive cardiovascular risk reduction in screen-detected diabetic patients Citation[12]. In the ADDITION Netherlands study, we performed a population-based screening in general practice from 2002 to 2004. All 56 978 non-diabetic patients, aged 50–70 years, from 79 general practices in the south-western region of the Netherlands, were invited to participate. The screening programme has been described elsewhere Citation[13]. The screening algorithm in the three countries was comparable, but to a certain extent country specific Citation[14]. The first step of the screening consisted of a mail-distributed, self-completed questionnaire, which contained questions about age, gender, body mass index, family history of diabetes, frequent thirst, use of antihypertensive medication, shortness of breath, claudication and cycling Citation[15]. The range of the scores was 0–29 points. People who scored above a predefined threshold (≥6 points) were considered to have increased risk of having diabetes and were invited to undergo subsequent diagnostic glucose testing. Actually, we performed a four-step screening procedure (questionnaire, random glucose measurement, fasting glucose measurement, oral glucose tolerance test) and a three-step procedure (without random glucose measurement). Diagnosis of diabetes was based on two diabetic glucose values. Participants were classified according to the 1999 World Health Organization criteria Citation[16]. Eventually, we detected 586 (1.0%) new diabetic patients Citation[13].
Practices and general practitioners
A questionnaire was distributed to all 106 GPs, who were employed in the 79 participating practices (37 single-handed, 42 group practices) and contained items on the GP age, gender, employment (full-time, part-time), special interest in diabetes (yes, no), providing insulin therapy in general practice (yes, no) and the practice setting (single-handed, group), location (urban, rural), number of patients from ethnic minority groups, specific diabetes clinic (yes, no), involvement of mainly practice assistant or practice nurse in diabetes care (yes, no), structured cooperation with a specialized diabetes nurse (yes, no). In the Netherlands, diabetes nurses, practice nurses or practice assistants (with a lower grade of professional education) are involved in diabetes care in general practice. GPs reported the prevalence of known diabetes in the practice. To improve the response rate, we reminded by telephone all GPs who did not return the questionnaire within 2 weeks. In the Netherlands, practically the entire population is registered with a GP. Within geographical borders, patients are free to choose one's family doctor.
Data analysis
To compare the yield of screening between practices, we calculated the ratio screen-detected diabetic patients/known diabetic patients (SDM/KDM) per practice. Furthermore, we adjusted the number of screen-detected diabetic patients per practice for practice size and for the proportion of people in the practice aged 50–70 years. In the Netherlands, 2350 registered patients per practice is considered normative (1.0 full-time equivalent). In the Dutch population, the proportion of people aged 50–70 is 22.6% Citation[17]. So we calculated the number of screen-detected diabetic patients per standardized practice (SDM per standardized practice): (2350/number of patients aged 50–70)×0.226×detected diabetic patients. The ratio SDM/KDM is likely to be more informative than SDM per standardized practice because in this ratio unknown factors are integrated, such as previous screening activities.
Of the 42 group practices, 19 were characterized by a very close professional cooperation between the GPs including the organization of daily diabetes care. In some of these practices, patients were not registered with one single GP. From these 19 group practices, only the characteristics of one GP, who was responsible for diabetes management, were taken into the analysis. The other 23 group practices were analysed as single-handed because such cooperation between GPs did not exist.
Statistical analyses were performed using SPSS for Windows (version 11.0). The associations between the yield of the screening and characteristics of GPs and practices were studied using linear regression analysis. Stepwise backward multiple regression analysis was performed to identify independent predictors of the yield of screening. Residuals were analysed to verify that the assumptions of linear regression were valid. A p-value <0.05 was considered significant.
Results
The prevalence of diabetes (in all age groups) before the screening in the practices was 3.1%, which is similar to the prevalence of diabetes in the Netherlands Citation[18]. The prevalences in urban and rural practices before screening did not differ (3.1% and 3.2%, respectively, p=0.87). Additionally, the prevalence of diabetes in the practices was compared between subregions (practices closer or more distant to the laboratory) and no significant differences were found (data not shown). The number of patients aged 50–70 years as a proportion of all practice patients was 22.1%, which is similar to the percentage in the Dutch population. In urban practices 29.2% of the invited individuals attended the screening, whereas in rural practices 32.7% attended (p=0.10). Mean (±SD) age of the screen-detected type 2 diabetic patients was 60.3±5.3 years.
We received completed questionnaires from all practices. shows the characteristics of the GPs and practices. Approximately half of the GPs reported to have special interest in diabetes. More than 70% of the GPs were providing insulin therapy in their practice. In a great majority of the practices, the population was predominantly Caucasian.
Table I. Characteristics of general practitioners and practices.
SDM per standardized practice ranged from 1.1 to 14.1 (mean 5.3, SD±2.7) (). In approximately 40% of the practices, the number of screen-detected diabetic patients varied between three and six persons. SDM/KDM varied between practices from 0.8% to 20.0% (mean 7.5%, SD±4.5).
Figure 1. The yield of screening in the 79 general practices. SDM/KDM, ratio screen-detected diabetic patients/known diabetic patients per practice; SDM per standardized practice, number of detected diabetic patients per practice after adjustment for practice size and age distribution.
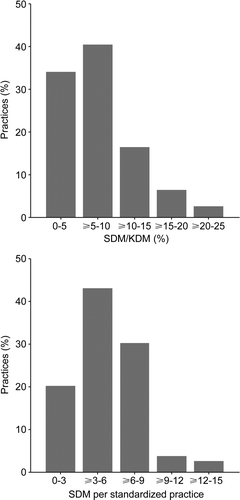
Univariate regression analyses with either SDM/ KDM or SDM per standardized practice as the dependent variable, demonstrated a significant association only with urban practice location and age of the GP (). Subsequently, the variables from were entered in a multivariate model. In stepwise backward linear regression analysis with SDM/KDM as the dependent variable, age, urban location and involvement of the practice assistant remained in the model (). In addition, structured cooperation with a diabetes nurse remained in the model when SDM per standardized practice was the dependent variable. In the first multivariate model R2 adjusted was 0.29 and in the second R2 adjusted was 0.25.
Table II. Associations of general practitioner and practice characteristics with both SDM/KDM and SDM per standardized practice.
Table III. Multivariate linear regression models of determinants of both outcome variables.
Discussion
Our study showed a wide variation in the number of detected diabetic patients per practice. The yield of screening was lower in practices of younger GPs and in urban practices and higher when the practice assistant participated in diabetes care. It is assum-able that a lower yield reflects a larger number of already diagnosed diabetic patients in the practice. Neither an appropriate practice organization, nor special interest or skills of the GP regarding diabetes care were associated with a lower yield. However, we should realize that the confidence intervals are generally wide making it difficult to draw very firm conclusions.
The association between the yield of screening and age of the GP seems in line with other study results. Aubin et al. found that younger physicians were more likely to appropriately screen for hypertension Citation[19]. In a systematic review evaluating the relationship between clinical experience and quality of care, it has been demonstrated that physicians in practice for more years were less likely to adhere to standards of practice for screening Citation[20]. Possibly younger GPs have been more trained for performing preventive tasks while older physicians may have less familiarity with these. On the other hand, Drivsholm et al. have recently suggested that GPs may diagnose diabetes at an earlier stage in patients they know well Citation[21]. How well the GPs knew their patients was assessed by how long the patient had been listed at the practice and by GPs’ subjective evaluation. Their finding seems to contrast with ours because it is plausible that older GPs know better, or at least longer, their patients than younger doctors.
Although structured cooperation with a diabetes nurse reduced the yield of screening, the strength of this association should not be overestimated. There was no significance when SDM/KDM was the dependent variable. Nevertheless, it is plausible that cooperation with a diabetes nurse increases diabetes awareness, resulting in a lower prevalence of undiagnosed diabetes and, consequently, a lower yield of screening. On the other hand, task delegation to practice assistants in diabetes care was associated with a higher yield. In the Netherlands, possibly task delegation to a practice assistant reflects a lesser diabetes awareness of the GP.
A limitation, which should be considered, is the unknown impact of the attendance rate on the yield of screening. We do not know the extent of the non-attendance nor its underlying reasons. There are two possible explanations for a low attendance rate. Firstly, participants do not attend the screening because their score on the questionnaire is below threshold. Secondly, people simply do not show up, although their score entitles them to undergo subsequent measurements. There are no reasons to expect a different behaviour of people in urban or rural areas in the same part of the country in this respect, but we cannot exclude such a difference. Therefore, the lower yield in urban practices might be explained by both a true lower prevalence of undiagnosed diabetes and by a larger proportion of subjects with scores above threshold who did not show up for further testing. A second limitation is that we made the assumption that a low yield of screening reflects a low prevalence of undiagnosed diabetes. As mentioned above, the unknown extent of non-attendance might have influenced the yield. Moreover, all people with impaired glucose regulation, including those with type 2 diabetes, tend to commute across the borders of diagnosis. On the other hand, the prevalence of diabetes before the screening in the practices was similar to the prevalence of diabetes in the Netherlands and neither differed between urban and rural practices, nor between practices closer or more distant to the laboratory. In urban and rural practices, a similar proportion of the invited individuals attended the screening. Interpreting a low yield as a reflection of a low prevalence of undiagnosed diabetes may be allowed but should be done with caution. A third limitation is that, in 19 group practices, we only took the characteristics of the GP, responsible for the organization of the diabetes care, into the analyses. However, we know that these GPs actually determined the profile of the practice regarding diabetes care (for instance because of providing insulin therapy only by this GP). Furthermore, GPs who were taken into the analyses and those who were not did not differ significantly with regard to age and gender (data not shown). Finally, older GPs might have a higher proportion of elderly patients, which might result in a higher yield of screening. However, if we compared the percentages of patients aged 50–70 years between practices of younger (≤50 years) and older (>50 years) GPs, similar proportions were found (23.4% and 24.3%, respectively, p=0.55).
We had expected to find an inverse relationship between the yield of screening and certain properties of the GP and practices such as special interest in diabetes or a specific diabetes clinic. However, this relationship was not found. Evidently, such properties do not lead automatically to early detection of undiagnosed diabetes. Detecting unidentified diabetic patients might require a well-structured screening programme. Our findings stress the importance of a proactive, systematic diabetes screening programme in all practices. Attention should be drawn to the fact that even in practices with an appropriate organization of diabetes care a screening strategy is indicated. In particular, this should be pointed out to older GPs.
In conclusion, a lower yield of screening, plausibly reflecting a lower prevalence of undiagnosed diabetes in the practice, was neither associated with a more appropriate practice organization regarding diabetes care nor with a specialty of the GP in diabetes. Younger GPs might perform better in detecting diabetic patients. The wide variation in the yield of screening emphasizes the importance of a healthcare provider initiated screening programme in general practice.
Competing interests
None declared.
Acknowledgements
We are grateful to the GPs who were willing to complete the questionnaire.
References
- Borch-Johnsen K, Lauritzen T, Glümer C, Sandbaek A. Screening for type 2 diabetes – Should it be now?. Diabet Med. 2003; 20: 175–181
- American Diabetes Association. Screening for type 2 diabetes (Position Statement). Diabetes Care 2004;27 Suppl 1, S11–S14.
- Engelgau MM, Narayan KM, Herman WH. Screening for type 2 diabetes. Diabetes Care. 2000; 23: 1563–1580
- Rutten GEHM, Verhoeven S, Heine RJ, De Grauw WJC, Cromme PVM, Reenders K, et al. NHG-standaard Diabetes Mellitus type 2 [Dutch College of General Practitioners’ guidelines on Type 2 diabetes mellitus). Huisarts Wet. 1999; 42: 67–84
- Wareham NJ, Griffin SJ. Should we screen for Type 2 diabetes? Evaluation against National Screening Committee criteria. Br Med J. 2001; 322: 986–988
- Greaves CJ, Stead JW, Hattersley AT, Ewings P, Brown P, Evans PH. A simple pragmatic system for detecting new cases of type 2 diabetes and impaired fasting glycaemia in primary care. Fam Pract. 2004; 21: 57–62
- Valk GD, Renders CM, Kriegsman DM, Newton KM, Twisk JW, Van Eijk JT, et al. Quality of care or patients with type 2 diabetes mellitus in the Netherlands and the United States: A comparison of two quality improvement programs. Health Serv Res. 2004; 39: 709–725
- Renders CM, Valk GD, Franse LV, Schellevis FG, Van Eijk JT, Van der Wal G. Long-term effectiveness of a quality improvement program for patients with type 2 diabetes in general practice. Diabetes Care. 2001; 24: 1365–1370
- Renders CM, Valk GD, Griffin SJ, Wagner EH, Van Eijk JT, Assendelft WJ. Interventions to improve the management of diabetes in primary care, outpatient, and community settings: A systematic review. Diabetes Care. 2001; 24: 1821–1833
- De Berardis G, Pellegrini F, Franciosi M, Belfiglio M, Di Nardo B, Greenfield S, et al. QuED Study. Quality of care and outcomes in type 2 diabetic patients: A comparison between general practice and diabetes clinics. Diabetes Care. 2004; 27: 398–406
- Hansen LJ, Olivarius N, de F, Siersma V, Andersen JS. Doctors’ characteristics do not predict long-term glycaemic control in type 2 diabetic patients. Br J Gen Pract. 2003; 53: 47–49
- Lauritzen T, Griffin S, Borch-Johnsen K, Wareham NJ, Wolffenbuttel BHR, Rutten GEHM. The ADDITION study: Proposed trial of the cost-effectiveness of an intensive multifactorial intervention on morbidity and mortality among people with Type 2 diabetes detected by screening. Int J Obes Relat Metab Disord. 2000; 24: S6–S11
- Janssen PGH, Gorter KJ, Stolk RP, Rutten GEHM. Low yield of population-based screening for type 2 diabetes in the Netherlands. The ADDITION Netherlands study. Fam Pract 2007;24:555–61
- Rasmussen SS, Glümer C, Sandbaek A, Lauritzen T, Borch-Johnsen K. Progression from impaired fasting glucose and impaired glucose tolerance to diabetes in a high-risk screening programme in general practice: The ADDITION Study, Denmark. Diabetologia. 2007; 50: 293–297
- Ruige JB, de Neeling JN, Kostense PJ, Bouter LM, Heine RJ. Performance of an NIDDM screening questionnaire based on symptoms and risk factors. Diabetes Care. 1997; 20: 491–496
- World Health Organization. Definition, diagnosis and classi fication of diabetes mellitus and its complications. Report of a WHO Consultation. Part 1: Diagnosis and classification of diabetes mellitus. Geneva: World Health Organization 1999.
- Statistics Netherlands. Available at: http://www.cbs.nl. Accessed 15 April 2005.
- Gijsen R, Baan CA, Feskens EJM. Hoe vaak komt diabetes mellitus voor en hoeveel mensen sterven eraan? [How often does diabetes mellitus occur and how many people die from it?); 2003. Available at: http://www.rivm.nl/vtv/data/kompas/gezondheidstoestand/ziekte/suikerziekte/suikerziekte_omvang.htm. Accessed 16 September 2004.
- Aubin M, Vézina L, Fortin JP, Bernard PM. Effectiveness of a program to improve hypertension screening in primary care. CMAJ. 1994; 150: 509–515
- Choudry NK, Fletcher RH, Soumerai SB. Systematic review: The relationship between clinical experience and quality of health care. Ann Intern Med. 2005; 142: 260–273
- Drivsholm T, de Fine Olivarius N. General practitioners may diagnose type 2 diabetes mellitus at an early disease stage in patients they know well. Fam Pract. 2006; 23: 192–197