Abstract
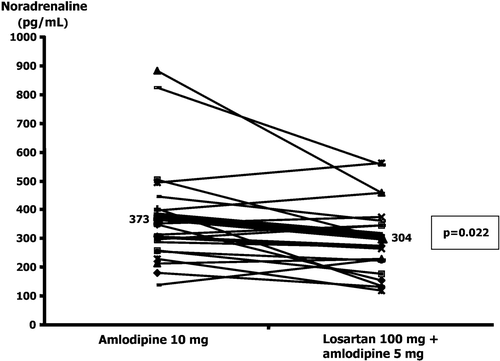
Aims. We have previously found improved insulin sensitivity in hypertensives after additional treatment with angiotensin II‐receptor blocker (ARB) compared with calcium‐channel blocker (CCB) alone, despite similar blood pressure lowering effects. In this study, we compare the effect of these two principal different vasodilating agents on the autonomic nervous system in the same patients, and test whether potential differences in these variables might explain the difference seen in insulin sensitivity. Methods. In a double‐blind crossover study, 21 hypertensive patients were randomized to receive either 100 mg losartan (ARB) or 5 mg amlodipine (CCB) in addition to an open‐labelled treatment of amlodipine 5 mg. The patients were treated for 8 weeks with either treatment regimens after a 4‐week run‐in and a 4‐week washout period. Plasma catecholamines were measured using radioenzymatic technique and baroreflex sensitivity and heart rate variability was tested at rest and during 24‐h ECG registration. Results. Plasma noradrenaline was significantly lower after additional treatment with ARB compared with CCB alone (304±29 pg/ml vs 373±43 pg/ml, p = 0.022). Heart rate variability, baroreflex sensitivity or plasma adrenaline did not differ significantly between the two treatment regimens. Conclusion. The results may suggest that improvement of insulin sensitivity by ARB is related to decreased plasma noradrenaline and potential sympatholytic effects.
Introduction
Reduction in new‐onset diabetes and improvements in insulin sensitivity by certain anti‐hypertensive drugs, primarily inhibitors of the renin–angiotensin system (RAS), have recently received much attention Citation[1], and to a degree this may be ascribed to the vasodilating effect of such drugs. However, there seems to be fewer cases of new‐onset diabetes in patients treated with blockers of RAS compared with calcium‐channel blockers (CCB) in large hypertension trials Citation[2–4], even though both treatment regimens are known to have vasodilating properties. Interestingly, one recent study, the VALUE trial, showed a 23% relative risk reduction of new‐onset diabetes on valsartan (angiotensin II‐receptor blocker, ARB) treatment compared with amlodipine (CCB) treatment Citation[4], Citation[5]. Since CCBs are considered neutral in their effects on glucose homeostasis Citation[1], Citation[6], this may indicate a possible preventive effect of the RAS‐blockade. We have previously compared the effects of two vasodilating agents, an ARB and a CCB, on peripheral insulin‐mediated glucose uptake in hypertensive patients with other cardiovascular risk factors. Insulin sensitivity assessed as glucose disposal rate (GDR) was shown to be significantly higher after treatment with losartan 100 mg+amlodipine 5 mg compared with treatment with amlodipine 10 mg, despite similar blood pressure lowering effects Citation[7]. Further investigations of this difference in insulin sensitivity showed no differences in levels of adipokines, viscosity and markers of inflammation and fibrinolysis in these patients Citation[8]. Thus, angiotensin II type 1 (AT1) receptor blockade seems to affect glucose metabolism beyond what can be expected by the vasodilatation and blood pressure (BP) reduction alone.
In the present study, we examined the effects of these two vasodilating treatment regimens on the autonomic nervous system in order to elucidate further possible mechanisms related to the difference seen in insulin sensitivity Citation[7]. Autonomic cardiac regulation can be evaluated by measurement of heart rate variability (HRV) Citation[9] and baroreflex sensitivity (BRS) Citation[10]. General sympathetic activity may be indirectly assessed by measurements of plasma catecholamines. We hypothesized that the two treatment regimens, despite similar changes in blood pressure, would have opposite effects on the autonomic nervous system and that such changes could explain the improvement of insulin sensitivity seen during the hyperinsulinaemic isoglycaemic glucose clamp in our study.
Patients and methods
Study design
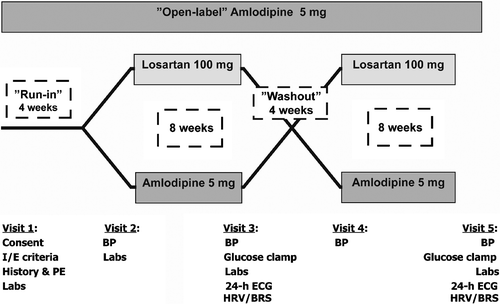
This was a 24‐week single centre, double‐blind, randomized crossover study as shown in Figure . After a 4‐week run‐in period with treatment with open‐label amlodipine 5 mg, all hypertensive patients (BP>140/90 mmHg) were randomized to either an addition of losartan 100 mg or amlodipine 5 mg for 8 weeks. At the end of this 8‐week period, patients underwent clinical examinations, laboratory tests, hyperinsulinaemic isoglycaemic glucose clamp and 24‐h ECG measurements. The patients then continued open‐label 5 mg amlodipine for a 4‐week washout period, before crossing over to the other treatment regimen for another 8 weeks as in the previous treatment period. Blood samples were collected from the patients after the two treatment periods (amlodipine 10 mg and losartan 100 mg+amlodipine 5 mg) and stored for later analyses of plasma catecholamine concentrations as one batch.
Biochemical methods and calculations
Blood samples were collected after overnight fasting and stored at −70°C prior to analyses. Plasma catecholamine concentrations were measured with a radioenzymatic technique Citation[11], which has been validated in our laboratory Citation[12].
The homeostasis model assessment for insulin resistance (HOMA‐IR) was calculated in fasting conditions as serum glucose (mmol/l) multiplied by serum insulin (pmol/l) and divided with 135, as described by Matthews and colleagues Citation[13]. A high HOMA‐IR denotes low insulin sensitivity or insulin resistance.
HRV and BRS
Before the clamp procedure, ECG and Finometer (Finometer®, Finapres Medical System, Amsterdam, The Netherlands) recording was started and R–R intervals were recorded continuously for 40 min during supine rest. HRV was computed using BeatScope (BeatScope®, Finapres Medical System) and Nevrokard (Nevrokard® Medistar, Ljubljana, Slovenia) software programs for short‐term recordings of 5 min at rest in supine position for frequency domain methods. As recommended by the Task Force of The European Society of Cardiology and The North American Society of Pacing and Electrophysiology Citation[9], the chosen short‐term, 5‐min recordings were free of missing data and with a minimum of ectopic beats and noise. Normalized high frequency (HF; 0.15–0.40 Hz) and low frequency (LF; 0.04–0.15 Hz), and LF/HF ratio were measured.
Twenty‐four‐hour Holter recordings were analysed with HRV time domain methods using Novacor Holter software (Novacor HolterSoft Ultima version 2.3.1, Cedex, France). The mean R–R interval, standard deviation of all normal R–R intervals (SDNN) and the percentage of R–R intervals differing by >50 ms from the preceding interval (PNN50) were analysed. The complete signal for both the short‐and long‐time evaluation was carefully edited by one of the authors (TAA) using visual checks and manual corrections of individual R–R intervals and QRS complex classification according to guidelines Citation[9].
Measurement of BRS was based on the beat‐to‐beat blood pressure and heart rate recordings performed with the finger blood pressure monitor (Finometer®, Finapres Medical System) on the third right finger and a Mingograph 7 recorder (Siemens Elema, Solna, Sweden) for concomitant analogue ECG recordings. Software from Nevrokard was used for the analyses of BRS in segments of 5 min.
Statistical analyses
The sample size of the study was calculated based on earlier studies of the effect of losartan on glucose metabolism by Moan et al. Citation[14], and the calculation was made according to our primary endpoint insulin sensitivity (GDR) and not to the secondary endpoints – plasma catecholamine concentrations, HRV and BRS – which are reported in the present investigation.
We used SPSS 14.0.1 (SPSS, Chicago, IL, USA) software for data management and statistical analysis. Changes over time were analysed using paired‐samples t‐test. Variables with a skewed distribution were analysed after logarithmical transformation, and were back‐transformed to natural units for presentation in text and tables. Non‐parametric analysis with Wilcoxon matched pairs signed rank sum test were used for non‐normally distributed data. A two‐tailed p‐value of 0.05 was considered the limit of statistical significance. All values are presented as mean±SEM unless stated otherwise. Carry‐over effects may occur in crossover designs and possible carry‐over effects are considered in the results.
Ethics
The National Committees for Research Ethics in Norway and the Norwegian Medicines Agency approved the study, and each patient gave verbal and written informed consent to participate before inclusion into the study. The first part of the study was partly financed by school grants given to us by Merck US. Merck (MSD, Norway) also supplied double‐blinded study medications (losartan 100 mg tablets and matching placebos, as well as amlodipine 5 mg and matching placebo), and open‐label 5 mg amlodipine capsules.
Results
Twenty‐one hypertensive patients (11 women and 10 men) with mean age 58.6 years (range 46–75 years) completed the study period. The study group had a mean body mass index of 29.2 kg/m2 and BP of 160±3/96±2 mmHg; 17 of the patients also completed two satisfactory hyperinsulinaemic isoglycaemic glucose clamps Citation[7], and 20 patients completed two satisfactory measurements of BRS and HRV registrations. BP was lowered to the same level after both treatment periods Citation[7]. GDR was significantly higher after treatment with losartan 100 mg+amlodipine 5 mg compared with amlodipine 10 mg (4.9±0.4 vs 4.2±0.5 mg/kg/min, p = 0.039), and there was a trend towards lower values of HOMA‐IR after losartan treatment (2.8±0.5 vs 3.1±0.6, p = 0.131), indicating improved insulin sensitivity after additional treatment with losartan 100 mg compared with amlodipine 5 mg as previously presented Citation[7].
There were no significant differences in resting heart rate during the visits. The heart rate was 66±2 beats/min at visit 1, and 68±2 and 69±2 beats/min when treated with amlodipine 10 mg and losartan 100 mg+amlodipine 5 mg, respectively. Plasma venous noradrenaline concentration showed significantly lower values after additional treatment with an ARB (Figure ), but no significant difference was found in adrenaline concentration. Eleven of the 20 patients who completed two measurements of plasma noradrenaline concentrations were treated with the losartan regimen in their first crossover period. Mean noradrenaline concentration on amlodipine 10 mg treatment was almost the same for the patients randomized to this treatment in their first crossover period (375±60 pg/ml, n = 9) and those randomized to this in the last crossover period (371±63 pg/ml, n = 11). The noradrenaline concentration on losartan 100 mg+amlodipine 5 mg was 291±46 pg/ml in the patients given the losartan treatment regimen in the first crossover period and 318±36 pg/ml in the patients given the losartan treatment regimen in the second crossover period, indicating no carry‐over effect.
HRV frequency domain and time domain analyses showed no significant differences between the two treatment regimens as shown in Table , and there were no significant differences found in analyses of BRS.
Table I. Heart rate variability and baroreflex sensitivity (mean±SEM).
Discussion
We found significantly improved insulin sensitivity as assessed by the hyperinsulinaemic isoglycaemic glucose clamp technique after treatment with losartan 100 mg+amlodipine 5 mg compared with treatment with amlodipine 10 mg, despite similar blood pressure reduction. Plasma noradrenaline was significantly lower after treatment with losartan 100 mg+amlodipine 5 mg compared with treatment with amlodipine 10 mg. HRV or BRS did not differ between the two treatment regimens.
According to the hypothesis of Julius et al. Citation[15], enhanced sympathetic activity is a primary factor associated with hypertension, insulin resistance and possibly obesity. Sympathetic stimulation can cause insulin resistance and insulin resistance can reciprocate sympathetic stimulation. A vicious cycle may evolve in which the components reinforce each other Citation[15]. Enhanced sympathetic tone may cause peripheral insulin resistance by β‐adrenergic receptor stimulation Citation[16], by conversion to more fast‐twitch insulin‐resistant muscle fibres Citation[17], by decreased capillary density Citation[18] and/or by α‐adrenergic vasoconstriction Citation[19–22]. Since treatment with amlodipine is associated with increased sympathetic activity Citation[23–28] and blockers of RAS may reduce sympathetic activation Citation[29], Citation[30], we speculate that the difference in insulin sensitivity seen in our study may be due to different effects on the sympathetic–parasympathetic balance. Our findings of significant lower venous plasma noradrenaline levels on ARB add supports to this assumption. Our findings are in accordance with a double‐blind placebo‐controlled crossover study of by Struck et al. Citation[24]. In their study, 18 hypertensive patients were treated with the CCB amlodipine or the ARB valsartan, and the CCB increased the level of plasma noradrenaline and muscle sympathetic nerve activity compared with both placebo and treatment with an ARB. The authors hypothesized that the difference was due to a shift in the baroreflex set‐point on AT‐1 blockade so that it operates in a more normotensive range, an effect absent with amlodipine treatment of similar duration Citation[24]. In a 16‐week open‐label parallel‐group study of obese hypertensives, treatment with the ARB valsartan significant reduced leptin, BMI and HOMA‐IR, while treatment with the CCB felodipine significantly increased noradrenaline Citation[31]. In the Candesartan Role on Obesity and on Sympathetic System (CROSS) study, treatment with the ARB candesartan cilexetil was compared with treatment with high‐dose hydrochlorothiazide in obese hypertensives Citation[30]. After a 12‐week period with similar blood pressure lowering effect between the regimens, there was significant improvement in insulin sensitivity in the patients treated with the ARB in addition to a reduction in sympathetic cardiovascular drive measured with direct microneurographic recording of muscle sympathetic nerve activity. There were, however, no changes in plasma catecholamines Citation[30].
No significant difference was found in the level of adrenaline between the two treatment regimens in our study. Other studies have also shown that increase in noradrenaline concentration with amlodipine is not associated with increase in adrenaline levels Citation[25], suggesting that there is a clear dissociation between the activation of sympathetic nerves and of the adrenal medulla. Further, in the analyses of HRV and BRS, no significant differences were found between the treatment regimens. Analysis of beat‐to‐beat HRV has emerged as a sensitive non‐invasive method to assess cardiac autonomic activity quantitatively Citation[32], Citation[33]. HRV has been found to be related to insulin sensitivity in young healthy subjects Citation[34], Citation[35] and hypertensive patients with insulin resistance have reduced HRV compared with other hypertensives and normotensives Citation[36]. In a previous study, treatment with losartan for 6 months improved HRV and BRS compared with treatment with the beta‐blocker atenolol in uncomplicated hypertensives Citation[37]. Impaired HRV at high frequencies (0.15–0.40 Hz) is primarily a marker of parasympathetic autonomic dysfunction, but spectral power in LF band (0.04–0.15 Hz) and blood pressure variability are also indices of sympathetic control. Although, no significant differences were noted after treatment with an ARB in our study population, there was a trend towards higher HF (representing vagal tone), and a lower LF (representing sympathetic with vagal tone) and therefore a lower LF/HF spectral power ratio was measured. This may suggest less sympathetic and more vagal drive after treatment with an ARB. A previous study has suggested that abnormalities in cardiac parasympathetic regulation precede impairment of blood vessel sympathetic control Citation[38], and since we expected differences between our treatment regimens primarily in the sympathetic nervous system, we may not see significant differences in HRV and BRS in our study at this point. Another potential mechanism may be that the effect of the ARB may be more on the peripheral turnover of noradrenaline than on the central sympathetic nervous system regulation. In our trial, the patients were treated with the losartan+amlodipine combination, and in a previous trial, it has been shown that ARB treatment did not attenuate the sympathetic activation induced by amlodipine Citation[28] and this may also explain the lack of significant results in our study.
Although heart rate is predominantly modulated by vagal activity Citation[39], elevated heart rate or tachycardia indicates increased sympathetic and decreased parasympathetic activity in hypertension. There is growing evidence that elevated heart rate is related to increased cardiovascular morbidity and mortality Citation[21], Citation[40], Citation[41], and heart rate has also been included as a risk factor in recent hypertension guidelines from ESH/ESC Citation[42]. A study by Facchini et al. has shown strong correlates between insulin resistance measured with insulin suppression test and heart rate Citation[43]. Previous studies by our group have shown negative correlations between insulin sensitivity measured with the glucose clamp and plasma catecholamine levels and heart rate even in healthy, young men Citation[44], Citation[45]. However, heart rate is a rather rough measurement of activity in the autonomic nervous system, and this together with being predominantly a parasympathetic measure, may explain why no difference in heart rate was found between our two treatment regimens.
There are some limitations of our study. The sample size of 22 patients was made on calculations of expected difference in insulin sensitivity between the two treatment regimens, and not on the secondary endpoints of plasma catecholamine concentrations, HRV and BRS. This has implications for power, and may explain the lack of significant difference in the HRV and BRS analyses. Power calculations according to the standard deviations in our HRV and BRS analyses estimates that we would have needed a sample of 30–200 to find a significant difference in the different HRV analyses. It has also been claimed that it takes more than 3 months treatment to show a beneficial effect on BRS and HRV after ARB treatment Citation[37], and our study included only 8‐week treatment periods. A disadvantage of the crossover design is that every patient has to complete both treatment periods and both measurements to be included in the analyses, and unfortunately, we lost one patient because of technical problems with the catecholamine analyses and the HRV/BRS analyses. The two treatment periods were separated by a 4‐week washout period intended to minimize carry‐over effects, and the order in which the drugs were given apparently had no effect on noradrenaline concentration during treatment with amlodipine 10 mg. However, noradrenaline on treatment with losartan 100 mg+amlodipine 5 mg was higher in the patients who were given losartan in the last crossover period, but the number of patients is too small to prove statistical significance (11 patients received losartan in the first crossover period, and nine in the second). We also measured catecholamine concentrations in venous and not arterial plasma due to ethical considerations. Probably, arterial samples are a more sensitive marker of overall sympathetic activity Citation[12], and better reflect sympathetic tone from heart and kidney because approximately 50% of peripheral venous noradrenaline is released from muscle sympathetic nerves Citation[46].
The RAS and the sympathetic nervous system are linked by a positive feedback relationship Citation[29], and therapeutic approaches that exert sympatho‐inhibitory effects have shown to improve insulin sensitivity Citation[47], Citation[48]. When hypertension is associated with metabolic disorders, such as obesity and diabetes, the degree of sympathetic activation appears to be potentiated Citation[49–51]. Thus a sympathetic overactivity could be expected in our patients. Sympatholytic effects of blockers of RAS may prevent or attenuate possible additional sympathetic activation induced by amlodipine Citation[52], and may explain the difference in metabolic function and improved insulin sensitivity seen after addition of an ARB in our study. The reduction seen in plasma noradrenaline may indicate a potential beneficial peripheral mechanism on the noradrenaline turnover. Furthermore, we believe that improvement of insulin sensitivity and possible reduction in diabetes development by blockers of the renin–angiotensin system may be related to the decreased plasma noradrenaline and potential sympatholytic effects.
Acknowledgements
The study was partly financed by two school grants of $55 000 given by Merck & Co., (New Jersey, USA) and Merck & Co. (Drammen, Norway) supplied study drugs. We thank Soneil Guptha, MD for administrative support in this context. S.E. Kjeldsen has served as a consultant and received grants from Merck & Co. and other major pharmaceutical companies.
We thank Anne Elise Larsen for analysing the venous catecholamines. And we are indebted to physicians and staff at Bentsebro legesenter, Collosseumklinikken, Ekeberg legekontor, Ellingsrud legesenter, L11 Familiehelsesenter, Manglerud legekontor, Nordseter legekontor, Nordstrand legekontor and Trosterud legekontor in Oslo for their kind help with recruiting patients.
References
- Elliott W. J., Meyer P. M. Incident diabetes in clinical trials of antihypertensive drugs: A network meta‐analysis. Lancet 2007; 369: 201–7
- Hansson L., Lindholm L. H., Ekbom T., Dahlöf B., Lanke J., Schersten B., et al. Randomised trial of old and new antihypertensive drugs in elderly patients: Cardiovascular mortality and morbidity the Swedish Trial in Old Patients with Hypertension‐2 study. Lancet 1999; 354: 751–1756
- Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002; 288: 2981–97
- Kjeldsen S. E., Julius S., Mancia G., McInnes G. T., Hua T., Weber M. A., et al. Effects of valsartan compared to amlodipine on preventing type 2 diabetes in high‐risk hypertensive patients: The VALUE trial. J Hypertens 2006; 24: 1405–12
- Julius S., Kjeldsen S. E., Weber M., Brunner H. R., Ekman S., Hansson L., et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: The VALUE randomised trial. Lancet 2004; 363: 2022–31
- Padwal R., Laupacis A. Antihypertensive therapy and incidence of type 2 diabetes: A systematic review. Diabetes Care 2004; 27: 247–55
- Aksnes T. A., Reims H. M., Guptha S., Moan A., Os I., Kjeldsen S. E. Improved insulin sensitivity with the angiotensin II‐receptor blocker losartan in patients with hypertension and other cardiovascular risk factors. J Hum Hypertens 2006; 20: 860–6
- Aksnes T. A., Seljeflot I., Torjesen P. A., Höieggen A., Moan A., Kjeldsen S. E. Improved insulin sensitivity by the angiotensin II‐receptor blocker losartan is not explained by adipokines, inflammatory markers, or whole blood viscosity. Metabolism 2007; 56: 1470–7
- Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J 1996; 7: 354–81
- La Rovere M. T., Mortara A., Schwartz P. J. Baroreflex sensitivity. J Cardiovasc Electrophysiol 1995; 6: 761–74
- Peuler J. D., Johnson G. A. Simultaneous single isotope radioenzymatic assay of plasma norepinephrine, epinephrine and dopamine. Life Sci 1977; 21: 625–36
- Kjeldsen S. E., Flaaten B., Eide I., Helgeland A., Leren P. Evidence of increased peripheral catecholamine release in patients with long‐standing, untreated essential hypertension. Scand J Clin Lab Invest 1982; 42: 217–23
- Matthews D. R., Hosker J. P., Rudenski A. S., Naylor B. A., Treacher D. F., Turner R. C. Homeostasis model assessment: Insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–9
- Moan A., Höieggen A., Seljeflot I., Risanger T., Arnesen H., Kjeldsen S. E. The effect of angiotensin II receptor antagonism with losartan on glucose metabolism and insulin sensitivity. J Hypertens 1996; 14: 1093–7
- Julius S., Valentini M., Palatini P. Overweight and hypertension: A 2‐way street?. Hypertension 2000; 35: 807–13
- Deibert D. C., DeFronzo R. A. Epinephrine‐induced insulin resistance in man. J Clin Invest 1980; 65: 717–21
- Zeman R. J., Ludemann R., Easton T. G., Etlinger J. D. Slow to fast alterations in skeletal muscle fibers caused by clenbuterol, a beta 2‐receptor agonist. Am J Physiol 1988; 254: E726–E732
- Brook R. D., Julius S. Autonomic imbalance, hypertension, and cardiovascular risk. Am J Hypertens 2000; 13: 112S–122S
- Jamerson K. A., Julius S., Gudbrandsson T., Andersson O., Brant D. O. Reflex sympathetic activation induces acute insulin resistance in the human forearm. Hypertension 1993; 21: 618–23
- Jamerson K. A., Smith S. D., Amerena J. V., Grant E., Julius S. Vasoconstriction with norepinephrine causes less forearm insulin resistance than a reflex sympathetic vasoconstriction. Hypertension 1994; 23: 1006–11
- Palatini P., Julius S. Heart rate and the cardiovascular risk. J Hypertens 1997; 15: 3–17
- Julius S., Gudbrandsson T., Jamerson K., Tariq S. S., Andersson O. The hemodynamic link between insulin resistance and hypertension. J Hypertens 1991; 9: 983–6
- Lefrandt J. D., Heitmann J., Sevre K., Castellano M., Hausberg M., Fallon M., et al. The effects of dihydropyridine and phenylalkylamine calcium antagonist classes on autonomic function in hypertension: The VAMPHYRE study. Am J Hypertens 2001; 14: 1083–9
- Struck J., Muck P., Trubger D., Handrock R., Weidinger G., Dendorfer A., et al. Effects of selective angiotensin II receptor blockade on sympathetic nerve activity in primary hypertensive subjects. J Hypertens 2002; 20: 1143–9
- de Champlain J., Karas M., Nguyen P., Cartier P., Wistaff R., Toal C. B., et al. Different effects of nifedipine and amlodipine on circulating catecholamine levels in essential hypertensive patients. J Hypertens 1998; 16: 1357–69
- Fogari R., Zoppi A., Corradi L., Preti P., Malalamani G. D., Mugellini A. Effects of different dihydropyridine calcium antagonists on plasma norepinephrine in essential hypertension. J Hypertens 2000; 18: 1871–5
- Lopez L. M., Thorman A. D., Mehta J. L. Effects of amlodipine on blood pressure, heart rate, catecholamines, lipids and responses to adrenergic stimulus. Am J Cardiol 1990; 66: 1269–71
- de Champlain J., Karas M., Assouline L., Nadeau R., LeBlanc A. R., Dube B., et al. Effects of valsartan or amlodipine alone or in combination on plasma catecholamine levels at rest and during standing in hypertensive patients. J Clin Hypertens 2007; 9: 168–78
- Grassi G. Renin–angiotensin‐sympathetic crosstalks in hypertension: Reappraising the relevance of peripheral interactions. J Hypertens 2001; 19: 1713–6
- Grassi G., Seravalle G., Dell'Oro R., Trevano F. Q., Bombelli M., Scopelliti F., et al. Comparative effects of candesartan and hydrochlorothiazide on blood pressure, insulin sensitivity, and sympathetic drive in obese hypertensive individuals: Results of the CROSS study. J Hypertens 2003; 21: 1761–9
- Fogari R., Derosa G., Zoppi A., Rinaldi A., Lazzari P., Fogari E., et al. Comparison of the effects of valsartan and felodipine on plasma leptin and insulin sensitivity in hypertensive obese patients. Hypertens Res 2005; 28: 209–14
- Liao D., Carnethon M., Evans G. W., Cascio W. E., Heiss G. Lower heart rate variability is associated with the development of coronary heart disease in individuals with diabetes: The atherosclerosis risk in communities (ARIC) study. Diabetes 2002; 51: 3524–31
- Aronson D. Pharmacologic modulation of autonomic tone: Implications for the diabetic patient. Diabetologia 1997; 40: 476–81
- Flanagan D. E., Vaile J. C., Petley G. W., Moore V. M., Godsland I. F., Cockington R. A., et al. The autonomic control of heart rate and insulin resistance in young adults. J Clin Endocrinol Metab 1999; 84: 1263–7
- Lindmark S., Wiklund U., Bjerle P., Eriksson J. W. Does the autonomic nervous system play a role in the development of insulin resistance? A study on heart rate variability in first‐degree relatives of Type 2 diabetes patients and control subjects. Diabet Med 2003; 20: 399–405
- Pikkujamsa S. M., Huikuri H. V., Airaksinen K. E., Rantala A. O., Kauma H., Lilja M., et al. Heart rate variability and baroreflex sensitivity in hypertensive subjects with and without metabolic features of insulin resistance syndrome. Am J Hypertens 1998; 11: 523–31
- Chern C. M., Hsu H. Y., Hu H. H., Chen Y. Y., Hsu L. C., Chao A. C. Effects of atenolol and losartan on baroreflex sensitivity and heart rate variability in uncomplicated essential hypertension. J Cardiovasc Pharmacol 2006; 47: 169–74
- Javorka M., Javorkova J., Tonhajzerova I., Javorka K. Parasympathetic versus sympathetic control of the cardiovascular system in young patients with type 1 diabetes mellitus. Clin Physiol Funct Imaging 2005; 25: 270–4
- Grassi G., Vailati S., Bertinieri G., Seravalle G., Stella M. L., Dell'Oro R., et al. Heart rate as marker of sympathetic activity. J Hypertens 1998; 16: 1635–9
- Kannel W. B., Kannel C., Paffenbarger R. S Jr., Cupples L. A. Heart rate and cardiovascular mortality: The Framingham Study. Am Heart J 1987; 113: 1489–94
- Palatini P., Benetos A., Grassi G., Julius S., Kjeldsen S. E., Mancia G., et al. Identification and management of the hypertensive patient with elevated heart rate: Statement of a European Society of Hypertension Consensus Meeting. J Hypertens 2006; 24: 603–10
- Mancia G., De B. G., Dominiczak A., Cifkova R., Fagard R., Germano G., et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2007; 25: 1105–87
- Facchini F. S., Stoohs R. A., Reaven G. M. Enhanced sympathetic nervous system activity. The linchpin between insulin resistance, hyperinsulinemia, and heart rate. Am J Hypertens 1996; 9: 1013–7
- Moan A., Nordby G., Rostrup M., Eide I., Kjeldsen S. E. Insulin sensitivity, sympathetic activity, and cardiovascular reactivity in young men. Am J Hypertens 1995; 8: 268–75
- Reims H. M., Höieggen A., Fossum E., Rostrup M., Eide I., Kjeldsen S. E. Glucose disposal rates calculated from 60‐ to 90‐minute isoglycemic hyperinsulinemic glucose clamp correlate with cardiovascular risk factors in borderline hypertensive young men. Metabolism 2001; 50: 1175–80
- Kjeldsen S. E., Schork N. J., Leren P., Eide I. K. Arterial plasma norepinephrine correlates to blood pressure in middle‐aged men with sustained essential hypertension. Am Heart J 1989; 118: 775–81
- Grassi G. Sympathetic deactivation as a goal of nonpharmacologic and pharmacologic antihypertensive treatment: Rationale and options. Curr Hypertens Rep 2003; 5: 277–80
- Harsch I. A., Schahin S. P., Radespiel‐Troger M., Weintz O., Jahreiss H., Fuchs F. S., et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med 2004; 169: 156–62
- Huggett R. J., Scott E. M., Gilbey S. G., Stoker J. B., Mackintosh A. F., Mary D. A. Impact of type 2 diabetes mellitus on sympathetic neural mechanisms in hypertension. Circulation 2003; 108: 3097–101
- Grassi G., Seravalle G., Quarti‐Trevano F., Dell'Oro R., Bolla G., Mancia G. Effects of hypertension and obesity on the sympathetic activation of heart failure patients. Hypertension 2003; 42: 873–7
- Grassi G. Counteracting the sympathetic nervous system in essential hypertension. Curr Opin Nephrol Hypertens 2004; 13: 513–9
- Taddei S., Virdis A., Mattei P., Duranti P., Favilla S., Salvetti A. Vascular renin–angiotensin system and sympathetic nervous system activity in human hypertension. J Cardiovasc Pharmacol 1994; 23(Suppl 1)S9–S14