Abstract
We have previously shown that the angiotensin receptor blocker valsartan is associated with a lower incidence of new‐onset type 2 diabetes than that with the calcium‐channel antagonist amlodipine in the treatment of hypertensive patients at high cardiovascular risk. We have now investigated the benefits of valsartan vs amlodipine in patients of different categories of diabetogenic risk. Some 9995 patients without diabetes at onset participated in VALUE, with average follow‐up of 4.2 years. Predictors of new diabetes were analyzed by stepwise logistic regression. A diabetes risk score for each patient was calculated based on a multivariate model. The risk of developing new diabetes in quartiles of risk for the disease was calculated as an odds ratio (OR) with 95% confidence intervals (CI). New diabetes was reported in 580 (11.5%) patients on valsartan and in 718 (14.5%) patients on amlodipine (p<0.0001). There was a more than sevenfold rise in the development of new diabetes from the lowest to the highest quartile of risk. When study treatment was included in the risk model, the odds in favor of valsartan in preventing new diabetes progressively increased with higher risk. Fifty‐two (4.03%) patients developed diabetes on valsartan and 50 (4.14%) patients on amlodipine in the lowest quartile of risk, 73 (5.70%) patients on valsartan and 83 (6.81%) patients on amlodipine in the second quartile, and 126 (10.27%) patients on valsartan and 160 (12.58%) patients on amlodipine in the third quartile. The difference between treatments was highly significant in quartile 4 with 329 (26.68%) patients developing new diabetes on valsartan vs 425 (33.57%) patients on amlodipine (OR = 0.72, 95% CI 0.61–0.86, p = 0.0002). The number of patients needed for treatment for the duration of the trial in order to gain the benefit of valsartan over amlodipine in preventing one new case of diabetes was 43 in the third quartile and 15 in the fourth quartile of risk categories. We conclude that valsartan compared with amlodipine reduces the risk of developing diabetes mellitus, particularly in hypertensive patients with the highest susceptibility for development of diabetes.
Introduction
Diabetes mellitus, and in particular type 2 diabetes, is emerging as a major health problem, which tends to cluster with hypertension in individuals at high risk of cardiovascular disease Citation[1]. Hypertension is an insulin resistant state and hypertensive subjects have an exaggerated tendency to develop diabetes with aging Citation[2]. Since high blood pressure is encountered in 20% of the adult population Citation[3] and its management is a priority in preventing cardiovascular complications Citation[4], antihypertensive strategies, which attenuate the trend towards diabetes, might have major public health implications.
In large‐scale prospective outcomes trials, treatment with angiotensin‐converting enzyme (ACE) inhibitors, calcium‐channel antagonists (CCAs) or alpha‐blockers is associated with the development of type 2 diabetes less frequently than following therapy with diuretics and beta‐blockers Citation[5–10], drugs known to predispose to diabetes Citation[10], Citation[11]. In this respect, CCAs are considered metabolically neutral and less prone than diuretics or beta‐blockers to provoke diabetes Citation[8–10].
The Valsartan Antihypertensive Long‐term Use Evaluation (VALUE) Citation[13–15] was designed to compare cardiac outcomes between treatment regimens based on the angiotensin receptor blocker (ARB) valsartan and the CCA amlodipine in a population with essential hypertension at high risk of cardiac disease recruited by a specific predefined age‐, risk factor‐ and disease‐dependent algorithm. A total of 15 245 eligible patients in 31 countries were randomized. The VALUE results showed no difference between the two drug regimens in the primary composite cardiac endpoint rate or mortality Citation[16], Citation[17].
In a pre‐specified analysis, we also investigated the development of diabetes in the 9995 patients who were non‐diabetic at outset of VALUE to examine whether a treatment including an ARB could reduce the risk of type 2 diabetes compared with CCA‐based treatment Citation[18]. This was the first opportunity to formally compare effects of an inhibitor of the renin–angiotensin system with a CCA on the development of new‐onset diabetes; one previous study reported a numerical difference in favor of an ACE inhibitor Citation[10], but its design precluded a formal statistical comparison. We found new diabetes in 580 (11.5%) patients on valsartan and in 718 (14.5%) patients on amlodipine (p<0.0001) Citation[18]. The LIFE Study Citation[19] also showed a major difference in favor of an ARB, losartan, over the comparator, the beta‐blocker atenolol. The LIFE investigators analyzed predictors of new‐onset diabetes in their study and the benefits of losartan over atenolol in quartiles of risk based on the predictors for developing diabetes in the same study Citation[20]. In light of this, we have now investigated the benefits of valsartan over amlodipine in patients in quartiles of risk for developing diabetes, i.e. in different risk categories in the VALUE trial.
Methods
Study design, patients and treatment
The design of VALUE has been described in detail elsewhere Citation[13–15]. A total of 15 245 patients with treated or untreated hypertension (SBP/DBP⩾140/90 mmHg) were randomized to valsartan‐ or amlodipine‐based regimens. As 5250 patients had diabetes at baseline, 9995 patients were included in this study of development of new‐onset diabetes. Patients were followed for 4–6 years with regular visits. Upward‐titration of medication was implemented in five steps to reach a goal BP of <140/90 mmHg. First, the doses of double blind medication were doubled, to valsartan 160 and amlodipine 10 mg, respectively, then hydrochlorothiazide (HCTZ) was given as first add‐on treatment (12.5–25 mg daily) in both arms. Further antihypertensive drugs excluding other ARBs could be given to achieve BP control. ACE inhibitors or CCAs were allowed only if these drugs were clinically indicated for reasons other than hypertension.
Definition of study endpoints
At baseline Citation[13], Citation[14], diabetes was defined by 1985 WHO criteria (fasting glucose >7.8 mmol/l). In 1999, during the course of the study, a WHO working group changed the definition to a fasting glucose of ⩾7.0 mmol/l. Consequently the new‐onset diabetes in this report is defined as fasting glucose of ⩾7.0 mmol/l during the study in patients with glucose <7.0 mmol/l at entry. The new definition increased the number of diabetics at entry; 222 and 205 additional patients in the valsartan and amlodipine groups, respectively, were among the total of 5250 considered to have diabetes and were not included in the analysis.
During the blinded phase of VALUE, it became apparent, based on results of other studies, that antihypertensive agents had differential potentials to induce new‐onset diabetes Citation[5–10], Citation[19], and fasting blood glucose estimation was therefore included as mandatory at study end. Otherwise, information on new diabetes was collected prospectively throughout the study by scrutinizing the adverse event reports and by detecting anti‐diabetic drug usage in the concomitant medication database. Investigators were encouraged to use the new (1999) WHO criteria Citation[21] in diagnosing new‐onset diabetes reported as adverse events and this protocol was pre‐specified in a study newsletter. In order to detect new‐onset diabetes, we first excluded all patients who at entry were diagnosed as diabetics, received anti‐diabetic agents or had abnormal glucose levels. To detect new‐onset diabetes mellitus, the criteria described below were applied to the VALUE patients at risk of new‐onset diabetes. In the primary analysis, at least one of the following three criteria was used, but patients counted only once for the diagnosis of new diabetes:
We accepted a diagnosis of diabetes reported as an adverse event during the trial by investigators, who were strongly encouraged to use WHO 1999 criteria Citation[20]: fasting glucose ⩾7.0 mmol/l and/or ⩾11.1 mmol/l, 2 h after oral intake of 75 g glucose if venous plasma or serum, and/or ⩾12.2 mmol/l if capillary full blood, on two separate occasions.
We scrutinized study reports of concomitant medication for patients who were started on oral anti‐diabetic drug or insulin during the course of the trial. This database contained a detailed directory of drugs by both generic and trade names in all participating countries.
At the study end, a single venous blood sample was drawn for plasma or serum glucose determination in the central laboratory. A diagnosis of new‐onset diabetes was made if the patient was reported to be fasting and the glucose concentration was ⩾7.0 mmol/l.
Statistical analysis
In the treatment comparison, odds ratios and 95% confidence intervals were calculated for patients who were not diabetic at baseline. A two‐tailed p‐value of less than 0.05 was considered significant. Univariate and multivariate stepwise logistic regression analyses were then used to further define predictors of new diabetes as detailed previously Citation[22]. Predictors were considered significant at p<0.001. For each patient, the response variable, which we called risk score, was calculated based on the observed values of the predictors together with the regression estimates. Patients were then divided into risk quartiles based on their risk scores. The reason we pre‐specified to analyze in quartiles was because the LIFE Study investigators used quartiles Citation[20]. New‐onset diabetes in treatment comparisons was further analyzed for each risk quartile.
Results
VALUE randomized a total of 15 245 patients. As 5250 patients had diabetes based on WHO 1999 criteria at baseline, 9995 patients were eligible for new‐onset diabetes analysis. Of these, 5032 were in the valsartan arm and 4963 were in the amlodipine arm. There were no differences between the two groups in baseline demographics and blood pressures, qualifying risk factors, qualifying diseases and baseline medication (data not shown). All previous antihypertensive drugs but not aspirin or statins were discontinued at randomization. Somewhat more antihypertensive medication including open‐label diuretics were given in the valsartan arm compared with the amlodipine arm, while there was no difference for aspirin or statins (data not shown). New‐onset diabetes was detected in 1298 patients. Of these 580 (11.5%) patients were in the valsartan arm and in 718 (14.5%) patients were in the amlodipine arm (OR = 0.77, 95% CI 0.69–0.87, p<0.0001).
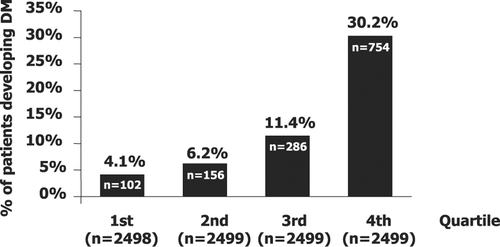
From the univariate logistic regression analyses, 14 of the 28 selected potential predictors of new diabetes were identified as significant (p<0.001). In order of significance, these were baseline blood glucose, body mass index (BMI), age, in‐study combined use of diuretic and beta‐blocker, in‐study use of diuretics, uric acid, race (white vs non‐white), hemoglobin, in‐study use of beta‐blocker, heart rate, in‐study potassium, randomized study treatment, history of coronary heart disease and male gender. In a multivariate model, which did not include randomized study treatment, significant predictors of new diabetes, in the order of χ2‐values (all p<0.001), were glucose, BMI, in‐study use of diuretic, white race, age and heart rate. We selected this model (Table ) for risk quartile analyses since it included all patients and it did not include randomized treatment as a potential predictor. The in‐study use of diuretic and beta‐blocker in combination was also a highly significant predictor, but was not included in the risk model since inclusion did not influence the results. In quartiles of subjects (n = 2499), number of patients with new diabetes increased from 102 (4.1%) in the lowest to 156 (6.2%) in the second, 286 (11.4%) in the third to 754 patients with new diabetes (30.2%) in the fourth quartile (Figure ). A total of 58% of patients who developed new‐onset diabetes were in risk quartile 4.
Table I. Means or percentages of components of the multivariate model of predictors of new‐onset diabetes according to quartiles of patients and ranked according to χ2‐values.
Figure 1 Number and percentage of patients who developed new‐onset type 2 diabetes mellitus in the quartiles of risk score according to the multivariate forward stepwise logistic regression model of predictors of diabetes.
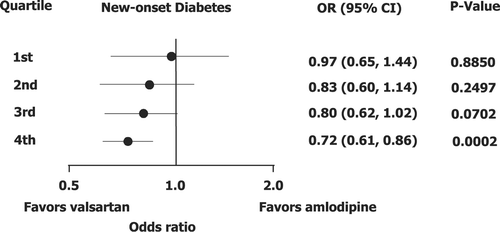
When study treatment was included into the risk model, the odds in favor of valsartan in preventing new diabetes increased progressively with higher risk (Table ). Fifty‐two (4.03%) patients developed diabetes on valsartan and 50 (4.14%) patients on amlodipine (OR = 0.97, 95% CI 0.65–1.44, p = 0.89) in the lowest quartile of risk, 73 (5.70%) patients on valsartan and 83 (6.81%) patients on amlodipine (OR = 0.83, 95% CI 0.60–1.14, p = 0.25) in the second quartile, and 126 (10.27%) patients on valsartan and 160 (12.58%) patients on amlodipine (OR = 0.80, 95% CI 0.62–1.02, p = 0.07) in the third quartile. The difference between treatments was highly significant in quartile 4 with 329 (26.68%) patients developing new diabetes on valsartan vs 425 (33.57%) patients on amlodipine (OR = 0.72, 95% CI 0.61–0.86, p = 0.0002, Figure ).
Table II. Quartile analysis of patients with new‐onset diabetes.
Figure 2 Point estimates for odds ratios(95% confidence intervals) for prevention of new‐onset type 2 diabetes mellitus on valsartan vs amlodipine in the quartiles of risk score developed according to the multivariate forward stepwise logistic regression model of predictors of diabetes.
The number of patients needed for treatment for the duration of the trial (NNT, 100 divided by % difference) in order to gain the befit of valsartan over amlodipine in preventing one new case of diabetes was 43 in the third quartile and 15 in the fourth quartile of risk categories (Table ).
Discussion
In this study of hypertensive patients, the ARB valsartan reduced the number of patients who developed new‐onset diabetes mellitus compared with the CCA amlodipine progressively with higher risk of developing the disease. The difference between treatment strategies was highly significant in the quartile of patients with the highest risk.
In the stepwise logistic regression models, the risk of developing diabetes in VALUE increased in an exponential fashion according to a risk score developed from six easily accessible variables, five at baseline plus the use of diuretic (with or without beta‐blocker) as add‐on treatment in the study. The incidence of new diabetes increased more than sevenfold from the lowest to the highest quartile of the risk score. Prevention of new diabetes by valsartan was marked in the three highest quartiles of diabetes risk and highly significantly so in the highest quartile. These findings provide a powerful tool for management of patients with hypertension and high cardiovascular risk in whom prevention of diabetes must be considered an important therapeutic goal. Without adjusting for whether subjects stayed on randomized treatment, the number of patients needed for treatment to prevent one patient from developing new‐onset diabetes for the duration of the study was 15 in the quartile with the highest diabetes risk and 43 in the quartile with the second highest risk. In clinical terms, the typical VALUE patient at high risk for developing new‐onset diabetes, and who might gain particular benefit from valsartan, is characterized by one or more of: fasting glucose 5–7 mmol/l, BMI>26 kg/m2, use of concomitant diuretic (with or without beta‐blocker), age below 70 years, non‐Caucasian race and relative tachycardia. These predictors have previously been discussed in depth Citation[22]. A potential limitation is lack of waist circumference though body build was assessed by BMI.
The change of the WHO criteria during the trial necessitated a change in the definition of diabetes at baseline. By altering the criterion from fasting glucose >7.8 to ⩾7.0 mmol/l, an additional 427 patients were identified as diabetic at baseline, 222 and 205 patients in the valsartan and amlodipine groups, respectively. Thus, the number of patients with diabetes at baseline is at variance with the report of patient's characteristics at baseline in VALUE Citation[14]. This correction was implemented for accuracy; except for potential under‐reporting of new cases during the trial, because of non‐adherence to the new criteria, it had no influence on the differential drug effects (data not shown). However, the change in criterion makes between‐study comparisons of total rates difficult. Diagnostic criteria have been less well defined in most other studies Citation[5–10] with the exception of LIFE Citation[18], Citation[19] where extensive investigations for new‐onset diabetes using WHO 1985 criteria were performed. The VALUE criteria were less stringent inasmuch as new drug treatment for diabetes was accepted assuming correct diagnosis.
To date, all prospective randomized outcome studies in hypertension have shown less new‐onset diabetes mellitus in the group receiving an agent that blocks the renin–angiotensin system, either ACE inhibitors or ARBs, than in the comparator group. Hitherto the comparator has been a diuretic Citation[10–15], Citation[23] or a beta‐blocker Citation[5], Citation[19] often in combination. VALUE provides the first formal comparison of new‐onset diabetes rates between an inhibitor of the renin–angiotensin system and a CCA, and the first direct comparison of an ARB and a CCA. This distinction of VALUE from other trials is important, since prior trials compared inhibitors of the renin–angiotensin system with diuretics and/or beta‐blockers. Both these classes of antihypertensive drugs negatively affect glucose balance Citation[10].
Treatment with diuretics, beta‐blockers or combination were predictors of new‐onset diabetes in VALUE; however, these drugs were given more often in the valsartan arm compared with the amlodipine arm in order to achieve blood pressure control, and particularly in those with higher blood pressure and higher risk. Despite this, the valsartan benefit over amlodipine was amplified with the higher risk and it was highly significant in the fourth quartile of risk score. Furthermore, as amlodipine lowered blood pressures more than valsartan in VALUE, somewhat contradictory to the neutral primary endpoint of cardiac morbidity and mortality, we may speculate that favorable metabolic effects of valsartan have balanced out the difference in blood pressure.
The potential benefit of ACE inhibitor was recently studied in the DREAM trial of people with high risk of developing diabetes; however, DREAM may have been underpowered for new‐onset diabetes as primary outcome Citation[24]. Possibly, participants would need to have hypertension in order to gain benefit of inhibiting the renin–angiotensin system in this respect. It is noteworthy, that blood glucose levels were significantly reduced in the ramipril arm of DREAM Citation[24]. The ongoing NAVIGATOR study, which is investigating the effect of valsartan in more than 9000 randomized participants, the majority with hypertension Citation[25], should provide further important information on the role of blockade of the renin–angiotensin system in attenuating the risk of new‐onset diabetes.
CCAs are considered metabolically neutral and a recent meta‐analysis Citation[11] suggests that these drugs, too, are associated with lower rates of new‐onset diabetes in comparison with diuretics/beta blockers. The demonstration in VALUE that the ARB valsartan is associated with lower rates of new‐onset diabetes rates compared to the CCA amlodipine, strongly suggests that positive findings with drugs interfering with the renin–angiotensin system are related to a beneficial effect of these drugs on glucose metabolism. The mechanism responsible for this effect cannot be determined with certainty from the VALUE findings. Prevention of new diabetes may partly lie in the improvement of microcirculation and a better delivery of glucose and insulin to skeletal muscles Citation[26]. Thus, antihypertensive treatment with the ARB losartan improves insulin sensitivity compared with the CCB amlodipine for the same level of blood pressure control Citation[27]. An influence of sympathetic drive as an underlying pathophysiological mechanism Citation[26] is supported by the predictive impact of higher heart rate at baseline. A direct effect on the endocrine pancreas may also be involved, as saralasin increased pancreatic islet blood flow in an experimental model Citation[28]. It has also been proposed that blockade of the renin–angiotensin system promotes the recruitment and differentiation of adipocytes, which would counteract the ectopic deposition of lipids in other tissues, liver, muscle, pancreas, thereby improving insulin sensitivity and preventing type 2 diabetes Citation[29].
In the LIFE Study, the ARB losartan compared with the beta‐blocker atenolol prevented new‐onset diabetes in all quartiles of risk for the disease Citation[20]. Thus, the present VALUE trial data are somewhat at variance with the LIFE findings, since in VALUE we observed a progressively increasing benefit of the ARB with higher risk of developing new‐onset diabetes. The different results may potentially be explained by the usage of different comparator, i.e. the beta‐blocker atenolol in LIFE and the CCB amlodipine in VALUE. The beta‐blocker may negatively affect glucose balance Citation[10] and thus make the difference compared to the beneficial effect of the ARB more apparent even in the lower risk category. The overall beneficial effects of ARBs in this context are fully compatible with the results of a recent network meta‐analysis Citation[30].
In summary, we tested for the first time the hypothesis that an inhibitor of the renin–angiotensin system would be better than a metabolically neutral CCA for prevention of new‐onset diabetes in hypertension, and we found that valsartan prevented new‐onset type 2 diabetes mellitus compared with the CCA amlodipine in the treatment of hypertensive patients at high cardiovascular risk. This effect was most pronounced in patients at the highest risk of developing new‐onset diabetes.
Acknowledgements
The VALUE Trial was supported by Novartis. Julie Jones and Allen Hester of Novartis Pharma, Basel, Switzerland are acknowledged for statistical work, and Laurence Designe and Suki Kumpeson for programming.
References
- Howard B. V., Rodrigues B. L., Bennett P. H. Prevention Conference VI: Diabetes and Cardiovascular Disease: Writing Group I: epidemiology. Circulation 2002; 105: e132–e137
- Kannel W. B., Wilson P. W., Zhang T. J. The epidemiology of impaired glucose tolerance and hypertension. Am. Heart J 1991; 121: 1268–73
- Kearney P. M., Whelton M., Reynolds K., Muntner P., Whelton P. K., He J. Global burden of hypertension: Analysis of worldwide data. Lancet 2005; 365: 217–23
- Zimmet P., Alberti K. G. M. M., Shaw J. Global and societal implications of the diabetic epidemic. Nature 2001; 414: 782–7
- Hansson L., Lindholm L. H., Niskanen L., Lanke J., Hedner T., Niklason A., et al. Effect of angiotensin‐converting‐enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: The Captopril Prevention Project (CAPPP) randomised trial. Lancet 1999; 353: 611–6
- The Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin‐converting‐enzyme inhibitor, ramipril, on cardiovascular events in high‐risk patients. N Eng J Med 2000; 342: 145–53
- The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research groups. Major cardiovascular events in hypertensive patients randomized to doxazosin vs. chlorthalidone. The Antihypertensive and Lipid Lowering treatment to prevent Heart Attack Trial (ALLHAT). JAMA 2000; 283: 1967–75
- Hansson L., Hedner T., Lund‐Johansen P., Kjeldsen S. E., Lindholm L. H., Syvertsen J. O., et al. Randomised trial of effects of calcium antagonists compared with diuretics and β‐blockers on cardiovascular morbidity and mortality in hypertension: The Nordic Diltiazem (NORDIL) Study. Lancet 2000; 356: 359–65
- Brown M., Palmer C. R., Castaigne A., de Leeuw P. W., Mancia G., Rosenthal T., Ruilope L. M. Morbidity and mortality in patients randomized to double‐blind treatment with a long‐acting calcium‐channel blocker or diuretic in the International Nifedipine GITS study: Intervention as a Goal in Hypertension Treatment (INSIGHT). Lancet 2000; 356: 366–72
- The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research groups. Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002; 288: 2981–97
- Opie L. H., Schall R. Old antihypertensives and new diabetes. J Hypertens 2004; 22: 1453–8
- Mancia G., Grassi G., Zanchetti A. New onset diabetes and antihypertensive drugs. J Hypertens 2006; 24: 3–10
- Mann J., Julius S., for the VALUE Trial Group. The Valsartan Antihypertensive Long‐term Use Evaluation (VALUE) Trial of Cardiovascular Events in Hypertension. Rationale and Design. Blood Press 1998; 7: 176–83
- Kjeldsen S. E., Julius S., Brunner H. R., Hansson L., Henis M., Ekman S., et al. Characteristics of 15314 hypertensive patients at high coronary risk. The VALUE Trial. Blood Press 2001; 10: 83–91
- Julius S., Kjeldsen S. E., Brunner H. R., Hansson L., Platt F., Ekman S., , for the Value Trial, et al. VALUE Trial: Long‐term BP trends in 13,449 patients with hypertension and high cardiovascular risk. Am. J. Hypertens 2003; 16: 544–8
- Julius S., Kjeldsen S. E., Weber M. A., Brunner H. R., Ekman S., Hansson L., , for the VALUE trial group, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: The VALUE randomised trial. Lancet 2004; 363: 2022–31
- Weber M. A., Julius S., Kjeldsen S. E., Brunner H. R., Ekman S., Hansson L., , for the VALUE trial group, et al. Blood pressure dependent and independent effects of antihypertensive treatment on clinical events in the VALUE Trial. Lancet 2004; 363: 1047–9
- Kjeldsen S. E., Julius S., Mancia G., McInnes G. T., Hua T., Weber M. A., et al. Effects of valsartan compared to amlodipine on preventing type 2 diabetes mellitus in high‐risk hypertensives patients: The VALUE trial. J Hypertens 2006; 24: 1405–12
- Dahlöf B., Devereux R. B., Kjeldsen S. E., Julius S., Beevers G., de Faire U., et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): A randomised trial against atenolol. Lancet 2002; 359: 995–1003
- Lindholm L. H., Ibsen H., Borch‐Johnsen K., Hecht Olsen M., Wachtell K., Dahlöf B., et al. Risk of new‐onset diabetes in the Losartan Intervention For Endpoint reduction in hypertension study. J Hypertens 2002; 20: 1879–86
- WHO. Department of Non‐Communicable Disease Surveillance. WHO 1999 criteria for diagnosis of diabetes mellitus. WHO, Geneva 1999; 1–59
- Aksnes T. A., Rostrup M., Kjeldsen S. E., Störset Ø., Hua A., Julius S. Predictors of diabetes in high risk hypertensive patients in the Value Trial. J Human Hypertens 2008, in press
- Lithell H., Hansson L., Skoog I., Elmfeldt D., Hofman A., Olofsson B., , for the SCOPE Study Group, et al. The Study on Cognition and Prognosis in the Elderly (SCOPE): Principal results of a randomized double‐blind intervention trial. J. Hypertens 2003; 21: 875–86
- Bosch J., Yusuf S., Gerstein H. C., Pogue J., Sheridan P., Dagenais G., et al. Effect of ramipril on the incidence of diabetes. N Engl J Med 2006; 355: 1551–62
- NAVIGATOR information site. Available from http://www.bioportfolio.com/news/novartis1.htm. 2004/10/24
- Julius S., Gudbrandsson T., Jamerson K. A., Tariq S. S., Andersson O. The hemodynamic link between insulin resistance and hypertension. J. Hypertens 1991; 9: 983–6
- Aksnes T. A., Reims H., Gupta S., Moan A., Os I., Kjeldsen S. E. Improved insulin sensitivity with the angiotensin II‐receptor blocker losartan in patients with hypertension and other cardiovascular risk factors. J Hum Hypertens 2006; 20: 860–6
- Carlsson P‐O., Berne C., Jansson L. Angiotensin II and the endocrine pancreas: Effects on islet blood flow and insulin secretion in rats. Diabetologia 1998; 41: 127–33
- Sharma A. M., Janke J., Gorzelniak K., Engeli S., Luft F. C. Angiotensin blockade prevents type 2 diabetes by formation of fat cells. Hypertension 2002; 40: 609–11
- Elliott W. J., Meyer P. M. Incident diabetes in clinical trials of antihypertensive drugs: A network meta‐analysis. Lancet 2007; 369: 201–7
Appendix
VALUE Committees
Executive Committee
Stevo Julius (Chair/USA), Sverre E. Kjeldsen (Associate Chair/Norway), Hans R. Brunner (Switzerland), John H. Laragh (USA), Gordon T. McInnes (UK), M. Anthony Schork (USA), Michael A. Weber (USA), Alberto Zanchetti (Italy), Lennart Hansson (Sweden).
Steering Committee
Felipe Martinez (Argentina), John Amerena (Australia), Dieter Magometschnigg (Austria), Jean‐Paul Degaute (Belgium), Wille Oigman (Brazil), Pierre Larochelle (Canada), Jun Ren Zhu (China), Jiri Widimský (Czech Republic), Ole Lederballe Pedersen (Denmark & Iceland), Silja Majahalme (Finland), Xavier Girerd (France), Roland E. Schmieder (Germany), Kostantinos Siamopoulos (Greece), Bela Herczeg (Hungary), Faustinus P. Rudyatmoko (Indonesia), Ian Graham (Ireland), Reuven Viskoper (Israel), Giuseppe Mancia (Italy), Cesar Calvo‐Vargas (Mexico), Nicolaas J. Holwerda (Netherlands), Morten Rostrup, Øyvind Størset (Norway), Andrezej Cieslinski (Poland), Ricardo Seabra Gomes (Portugal), Janna Kobalava (Russia), Ivan Balazovjech (Slovak Republic), Graham Cassel (South Africa), Antonio Coca (Spain), Edouard Battegay (Switzerland), Nevres Koylan (Turkey), Thomas MacDonald (UK), Kenneth Jamerson (USA).
Endpoint Committee
Luis M. Ruilope (Chair/Spain), Gregory Alberts (USA), Vivencio Barrios (Spain), Arie J. Man in't Veld (Belgium), Per Omvik (Norway), William Parmley (USA), Enrico Agabiti Rosei (Italy), Svend Strandgaard (Denmark), Myron H. Weinberger (USA).
Endpoint Coordinating Centre (Madrid, Spain)
Ana Garçia de Castro (Medical Back‐Up), Josefa Navarro, Alexandra Rissmann, Pilar Martinez Gutierrez, Isabel Martinez Gutierrez.
Data and Safety Monitoring Board
Stephen MacMahon (Chair/Australia), Henry R. Black (USA), Thomas Fleming (USA), Peter Sleight (UK).
Operations Committee
Stevo Julius (USA), Sverre E. Kjeldsen (Norway), Tsushung A. Hua (Novartis East Hanover, NJ, USA), Beverly Smith (Novartis East Hanover, NJ, USA), Deborah James (Novartis East Hanover, NJ, USA), Francis Plat (Novartis Basel, Switzerland), Steffan Ekman (Novartis Basel, Switzerland), Patrizia Kobi (Novartis Basel, Switzerland).