Abstract
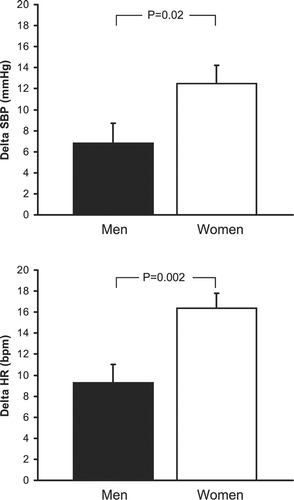
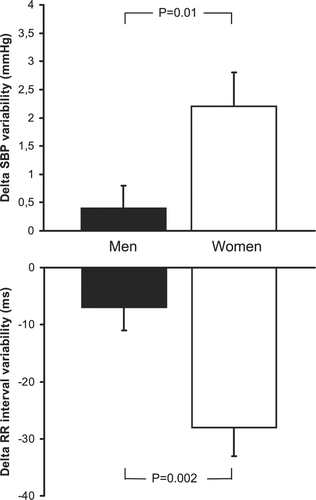
Objective. Smoking, a major risk factor for cardiovascular morbidity and mortality, may be particularly harmful to women. Sympathetic and hemodynamic responses to cigarette smoking may be implicated in the link between smoking and acute cardiovascular events. We tested the hypothesis that acute effects of smoking on cardiovascular function are potentiated in women compared with men. Methods. We examined the effects of cigarette smoking and sham smoking on muscle sympathetic nerve activity, blood pressure and heart rate in 20 female and 20 male middle‐aged healthy habitual smokers. Results. Sham smoking had no effect on muscle sympathetic nerve activity, blood pressure, or heart rate. Although cigarette smoking increased average systolic blood pressure and heart rate in both females and males, systolic blood pressure increased more in women (12±2 mmHg) than in men (6±2 mmHg; p = 0.02), as did heart rate (16±2 beats/min in women vs 9±2 beats/min in men; p = 0.002). Female smokers also had greater smoking‐related increases in systolic blood pressure variability compared with males (2.2±0.6 vs 0.4±0.4 mmHg, respectively; p = 0.01) and greater decreases in RR variability (−28±5 vs −7±4 ms; p = 0.002). Despite the potentiated blood pressure increase in females, which would be expected to inhibit sympathetic activity to a greater extent in females than in males, changes in muscle sympathetic nerve activity during smoking were similar in both sexes. Conclusions. Acute pressor and tachycardic effects of smoking are potentiated in women compared with men. These findings may have important implications for understanding increased vulnerability to acute cardiovascular events in women who smoke.
Introduction
Smoking remains a major risk factor for cardiovascular morbidity and mortality Citation[1], Citation[2]. Despite the protective effects of female gender against cardiovascular disease, smoking may be especially harmful to women Citation[3–8]. Middle‐aged female smokers have a 50% increase in relative risk for myocardial infarction than males Citation[5], and increased risk for vascular mortality Citation[6]. Mechanisms underlying differential effects of smoking on cardiovascular risk in females and males are unknown.
Acute effects of smoking in women are more important than chronic exposure in development of coronary thrombosis Citation[5]. Among smokers, cardiac ischemia is five times more likely when patients are actually smoking than when they are not smoking Citation[9]. Acute sympathetic and hemodynamic responses to cigarette smoking may be implicated in the link between smoking and cardiovascular events. Autonomic cardiovascular control is differentially affected by gender Citation[10–13]. Whether autonomic and hemodynamic responses to smoking are gender‐dependent has not been previously been studied. We therefore tested the hypothesis that the acute circulatory responses to smoking are potentiated in women compared with men.
Methods
Subjects
We studied 20 pre‐menopausal female and 20 male habitual smokers smoking 10–20 cigarettes per day for more than 2 years. Subjects were recruited from Gdansk community. Both groups were otherwise healthy, and none of the subjects was taking any medication nor had any history of chronic disease. Both groups had similar age (47±3 vs 46±3 years, respectively; mean±SEM), body mass index (26±1 vs 26±1 kg/m2, respectively) and duration of smoking habit (24±2 vs 27±3 years, respectively), and smoked a similar number of cigarettes per day (13±2 vs 15±2 cigarettes per day, respectively). The studies were approved by the Institutional Review Board on Human Investigation and written informed consent was obtained.
Measurements
Subjects were studied in the supine position. Heart rate was measured continuously by an electrocardiogram (ECG; AD Instruments). Blood pressure was measured continuously by the beat‐by‐beat Finapres system. Multiunit postganglionic sympathetic nerve activity was recorded using tungsten microelectrodes (shaft diameter 200 µm, tapering to an uninsulated tip of 1–5 µm) inserted selectively into muscle fascicles of the peroneal nerve. A subcutaneous reference electrode was first inserted 2–3 cm away from the recording electrode, which was itself inserted into the nerve fascicle. The neural signals were amplified, filtered, rectified and integrated to obtain a voltage display of sympathetic nerve activity.
Protocol and procedures
All subjects were asked to avoid smoking for at least 12 h prior to each study. Subjects were studied in the supine position. The study design was randomized and placebo‐controlled with two experimental sessions (sham smoking and smoking). The sessions were performed in random order, each session on a separate day.
After 15 min of rest, baseline measurements were obtained. The subjects were then asked to smoke two cigarettes each containing 1.1 mg nicotine or asked to simulate smoking using a drinking straw with a filter (sham smoking). The two cigarettes were separated by 5 min.
Analyses
Tracings of ECG, blood pressure and muscle sympathetic nerve activity (MSNA) were recorded with the PowerLab data acquisition system (AD Instruments). The amplitude of each burst was determined and muscle sympathetic activity was calculated as bursts/min, multiplied by mean burst amplitude and expressed as units/min. Baseline measurements of nerve activity, heart rate and blood pressure were obtained during 10 min rest before smoking cigarettes. Changes in sympathetic nerve activity, systolic blood pressure and heart rate were calculated for the last 3 min of smoking the second cigarette. MSNA was expressed as a percentage of change from baseline. Standard deviation of systolic blood pressure and RR interval were calculated to assess blood pressure Citation[14] and heart rate variability Citation[15].
For statistical analysis, we used Statistica software, version 8.0 (StatSoft Inc., Tulsa, OK, USA). Results are expressed as means±SEM. Responses to smoking were analyzed by repeated‐measures analysis of variance (ANOVA) with time (before vs during smoking) as within factor and group (males vs females) as between factor. The p‐values for differences within a session were obtained by post hoc tests. The key variable was the group‐by‐time interaction. A p<0.05 was considered significant.
Results
Male and female smokers were comparable for resting blood pressure, heart rate and MSNA (Table ). Resting blood pressure variability and RR variability were also similar (Table ).
Table I. Resting measurements in male and female habitual smokers.
Table illustrates hemodynamic and autonomic responses to sham smoking, which had no significant effect on blood pressure, heart rate or MSNA in either group (Table ).
Table II. Blood pressure, heart rate and muscle sympathetic nerve activity during sham smoking.
Cigarette smoking increased systolic blood pressure and heart rate in both female and male smokers (Table ). However, systolic blood pressure increased more strikingly in females (Figure , top), as did heart rate (Figure , bottom). In comparison with males, female smokers also showed more marked increases in systolic blood pressure variability (Figure , top) and decreases in RR variability (Figure , bottom). Despite the greater blood pressure increase in females, which would be expected to inhibit sympathetic activity to a greater extent in females than in males, changes in MSNA during smoking were similar in both genders (+5±6% vs +3±7%, respectively; p = NS).
Table III. Blood pressure, heart rate and muscle sympathetic nerve activity during cigarette smoking.
Discussion
These studies provide direct evidence that acute autonomic, pressor and tachycardic responses to smoking are potentiated in women compared with men. The novel and important findings include first, that increases in blood pressure and heart rate during smoking are greater in female smokers than in males. Second, smoking alters cardiovascular variability to a greater extent in females than males. Third, vasoconstrictor sympathetic tone is not suppressed in women during smoking despite the enhanced pressor response, so that sympathetic drive is inappropriately heightened in women during smoking.
The more potent pressor and tachycardic effects of smoking in women might help explain the greater impact of smoking on their cardiovascular and cerebrovascular morbidity Citation[3–7]. Cardiac ischemic events are far more likely to occur during actual smoking Citation[9]. Higher blood pressure Citation[16] increased blood pressure variability Citation[17], tachycardia Citation[18] and decreased heart rate variability Citation[19] have been linked to greater cardiac and vascular risk. Increased blood pressure variability in hypertensive patients is associated with an increased likelihood of target‐organ damage Citation[14], independent of the absolute blood pressure level. Transient decreases in heart rate variability may be especially important in the setting of cardiac ischemia, where it has been implicated in triggering fatal arrhythmia and sudden cardiac death Citation[20].
The increases in blood pressures in both genders would be expected to suppress sympathetic activity to levels approaching zero, and to slow heart rate. However, consistent with the sympatho‐excitatory Citation[21] and vagal inhibitory effects Citation[22] of cigarette smoking, sympathetic traffic remained inappropriately high. This was especially evident in women, in whom, despite the substantially greater blood pressure increase, sympathetic activity was not suppressed, but rather increased by 6%. Similarly, heart rates were inappropriately faster, especially in women, in whom the tachycardic response to cigarette smoking was about 60% greater than in men, despite the markedly higher blood pressures.
The mechanisms underlying the differential cardiovascular responses during smoking in females may include less effective baroreflex buffering of blood pressure in women than in men Citation[10]. Potentiated heart rate responses to smoking might also reflect increased β1‐receptor responsiveness in females Citation[11]. Finally, gender‐related differences in vagal mechanisms may be implicated. Smoking increases heart rate in part by reducing vagal restraint Citation[22]. Female subjects have higher levels of tonic vagal activity. The parasympathetic contribution to heart rate variability is greater in females than in males Citation[12], Citation[13]. Thus, smoking related inhibition of vagal activity, and consequent increases in heart rate and decreases in heart rate variability, would be more marked in women than in men.
The sympathetic nervous system has the capacity for selective changes in efferent discharge to different subdivisions. MSNA reflects only the vasoconstrictor signal to the skeletal muscle vasculature. Therefore, potential limitations of our study include the absence of additional measures of sympathetic tone such as plasma norepinephrine levels or regional norepinephrine spillover. With regard to plasma norepinephrine measurements, Grassi et al. Citation[23] have shown that reproducibility and sensitivity of this method is relatively poor in comparison with MSNA. Thus, it is unlikely that plasma norepinephrine measurements would provide any additional information. While the evaluation of regional norepinephrine spillover could provide new insights into the relationship smoking and cardiovascular function, these invasive measurements were not feasible in the large cohort we describe.
The worldwide prevalence of smoking among women is expected to rise continuously in the near future. Despite the protective effects of female gender on cardiovascular risk, smoking associated risk for ischemic cardiac events and stroke are as high or higher in women compared with men Citation[1–7]. Remarkably, however, the majority of women smokers perceive their lifetime risk for developing heart disease as average or below average Citation[24]. This may help explain why recent risk factor surveillance data from Europe suggest that while smoking continues a long‐term decline in men, it continues to increase in women Citation[25]. Cardiovascular risks of smoking are evident in women at levels of tobacco consumption that are only half as much as levels that increase cardiovascular risk in men Citation[6]. Heavy smoking in women is associated with a fourfold increased risk of sudden cardiac death, similar to that conferred by a history of myocardial infarction Citation[26]. Indeed, 41% of the coronary events in low‐risk females could be attributed to current smoking Citation[27]. In a study of almost 25 000 subjects, women 55 and younger, who were actively smoking, had a relative risk of acute myocardial infarction more than twofold greater than in men Citation[5]. The increased risk of smoking in women appears to be selective for cardiovascular disease, since cancer risk was not influence by gender Citation[6]. Acute neural circulatory responses may be implicated in the heightened smoking‐related cardiac and vascular risk in women.
Acknowledgement
Supported by NIH HL61560 and NIH FIRCA Award R03 TW0 1148. Potential conflict of interest: None.
References
- Ezzati M., Henley S. J., Thun M. J., Lopez A. D. Role of smoking in global and regional cardiovascular mortality. Circulation 2005; 112: 489–97
- Yusuf S., Hawken S., Ounpuu S., Dans T., Avezum A., Lanas F., et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case–control study. Lancet 2004; 364: 937–52
- McElduff P., Dobson A., Beaglehole R., Jackson R. Rapid reduction in coronary risk for those who quit cigarette smoking. Aust N Z J Public Health 1998; 22: 787–91
- Njolstad I., Arnesen E., Lund‐Larsen P. G. Smoking, serum lipids, blood pressure, and sex differences in myocardial infarction. A 12‐year follow‐up of the Finnmark Study. Circulation 1996; 93: 450–6
- Prescott E., Hippe M., Schnohr P., Hein H. O., Vestbo J. Smoking and risk of myocardial infarction in women and men: Longitudinal population study. BMJ 1998; 316: 1043–7
- Prescott E., Osler M., Andersen P. K., Hein H. O., Borch‐Johnsen K., Lange P., et al. Mortality in women and men in relation to smoking. Int J Epidemiol 1998; 27: 27–32
- Prescott E., Scharling H., Osler M., Schnohr P. Importance of light smoking and inhalation habits on risk of myocardial infarction and all cause mortality. A 22 year follow up of 12 149 men and women in The Copenhagen City Heart Study. J Epidemiol Community Health 2002; 56: 702–6
- Vriz O., Nesbitt S., Krause L., Majahalme S., Lu H., Julius S. Smoking is associated with higher cardiovascular risk in young women than in men: The Tecumseh Blood Pressure Study. J Hypertens 1997; 15: 127–34
- Gabbay F. H., Krantz D. S., Kop W. J., Hedges S. M., Klein J., Gottdiener J. S., et al. Triggers of myocardial ischemia during daily life in patients with coronary artery disease: Physical and mental activities, anger and smoking. J Am Coll Cardiol 1996; 27: 585–92
- Christou D. D., Jones P. P., Jordan J., Diedrich A., Robertson D., Seals D. R. Women have lower tonic autonomic support of arterial blood pressure and less effective baroreflex buffering than men. Circulation 2005; 111: 494–8
- Convertino V. A. Gender differences in autonomic functions associated with blood pressure regulation. Am J Physiol 1998; 275: R1909–20
- Evans J. M., Ziegler M. G., Patwardhan A. R., Ott J. B., Kim C. S., Leonelli F. M., et al. Gender differences in autonomic cardiovascular regulation: Spectral, hormonal, and hemodynamic indexes. J Appl Physiol 2001; 91: 2611–8
- Huikuri H. V., Pikkujamsa S. M., Airaksinen K. E., Ikaheimo M. J., Rantala A. O., Kauma H., et al. Sex‐related differences in autonomic modulation of heart rate in middle‐aged subjects. Circulation 1996; 94: 122–5
- Frattola A., Parati G., Cuspidi C., Albini F., Mancia G. Prognostic value of 24‐hour blood pressure variability. J Hypertens 1993; 11: 1133–7
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation 1996; 93: 1043–65
- Lewington S., Clarke R., Qizilbash N., Peto R., Collins R. Age‐specific relevance of usual blood pressure to vascular mortality: A meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360: 1903–13
- Parati G., Lantelme P. Blood pressure variability, target organ damage and cardiovascular events. J Hypertens 2002; 20: 1725–9
- Palatini P., Julius S. Heart rate and the cardiovascular risk. J Hypertens 1997; 15: 3–17
- Tsuji H., Larson M. G., Venditti F. J Jr., Manders E. S., Evans J. C., Feldman C. L., et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation 1996; 94: 2850–5
- Pozzati A., Pancaldi L. G., Di Pasquale G., Pinelli G., Bugiardini R. Transient sympathovagal imbalance triggers “ischemic” sudden death in patients undergoing electrocardiographic Holter monitoring. J Am Coll Cardiol 1996; 27: 847–52
- Narkiewicz K., van de Borne P. J., Hausberg M., Cooley R. L., Winniford M. D., Davison D. E., et al. Cigarette smoking increases sympathetic outflow in humans. Circulation 1998; 98: 528–34
- Niedermaier O. N., Smith M. L., Beightol L. A., Zukowska‐Grojec Z., Goldstein D. S., Eckberg D. L. Influence of cigarette smoking on human autonomic function. Circulation 1993; 88: 562–71
- Grassi G., Bolla G., Seravalle G., Turri C., Lanfranchi A., Mancia G. Comparison between reproducibility and sensitivity of muscle sympathetic nerve traffic and plasma noradrenaline in man. Clin Sci 1997; 92: 285–9
- Moran S., Glazier G., Armstrong K. Women smokers' perceptions of smoking‐related health risks. J Womens Health (Larchmt) 2003; 12: 363–71
- Costanza M. C., Salamun J., Lopez A. D., Morabia A. Gender differentials in the evolution of cigarette smoking habits in a general European adult population from 1993–2003. BMC Public Health 2006; 6: 130
- Albert C. M., Chae C. U., Grodstein F., Rose L. M., Rexrode K. M., Ruskin J. N., et al. Prospective study of sudden cardiac death among women in the United States. Circulation 2003; 107: 2096–101
- Stampfer M. J., Hu F. B., Manson J. E., Rimm E. B., Willett W. C. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med 2000; 343: 16–22