Abstract
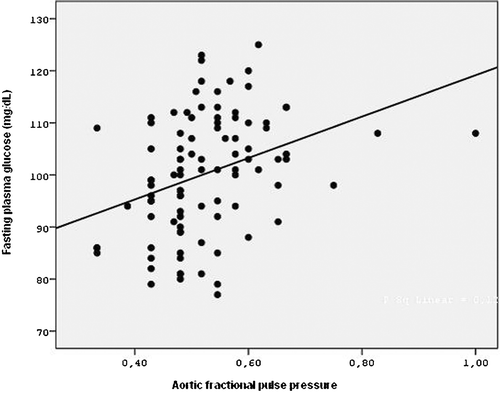
Background. Diabetes mellitus and impaired fasting glucose (IFG) are associated with future cardiovascular disorders. Aortic pulse pressure (PP) and fractional pulse pressures (FPPs) are strong and independent indicators of the risk of coronary heart disease. These conditions have been reported to be associated with endothelial dysfunction. In the present study, aortic PP and FPPs of patients with and without impaired fasting glucose were evaluated. Methods. Fifty patients with IFG with a mean age of 56.8±12.2 years and 47 patients with normal fasting glucose (NFG) with a mean age of 53.1±11.2 years were included in the study. All subjects had angiographically proven normal coronary arteries without coronary slow flow. Aortic systolic and diastolic blood pressures were measured invasively. Mean pressure, PP and FPPs (aortic PP/mean pressure) were calculated. Results. All parameters measured were significantly higher in the IFG group than in the control (NFG) group (133±21 mmHg and 117±12 mmHg, p<0.001 for aortic systolic pressure; 79±12 mmHg and 74±8 mmHg, p = 0.035 for aortic diastolic pressure; 97±14 mmHg and 88±9 mmHg, p = 0.001 for aortic mean pressure; 54±13 mmHg and 43±8 mmHg, p<0.001 for aortic PP; 0.56±0.10 and 0.48±0.08, p<0.001 for aortic FPP). In addition, in linear regression analysis, a positive correlation was found between fasting plasma glucose and the aortic FPP (p = 0.001, R2 = 0.12). Conclusion. Ascending aorta PP and FPPs are significantly associated with the presence of IFG. These findings suggest that IFG is associated with endothelial dysfunction and so aortic stiffness.
Introduction
Diabetes mellitus is an extensively studied and well known risk factor for the development of cardiovascular disorders. Impaired fasting glucose (IFG), defined as glucose levels of 100–125 mg/dl (5.6–6.9 mmol/l) in fasting patients, is accepted as a prediabetic condition that is also associated with future cardiovascular events Citation[1].
Pulse pressure (PP), the difference between systolic and diastolic blood pressures, has been shown to be associated with adverse cardiovascular events independently Citation[2], Citation[3]. In addition, aortic fractional pulse pressure (FPP) has been demonstrated to be associated with the presence and extent of coronary atherosclerosis in some studies Citation[4–6].
It is demonstrated that aortic stiffness is associated with diabetes mellitus Citation[7]. Is there a causative relationship present between IFG and aortic PP/FPPs?
The aim of the present study was to assess aortic PP/FPPs in subjects with IFG, compared with well‐matched healthy controls.
Methods
Study population
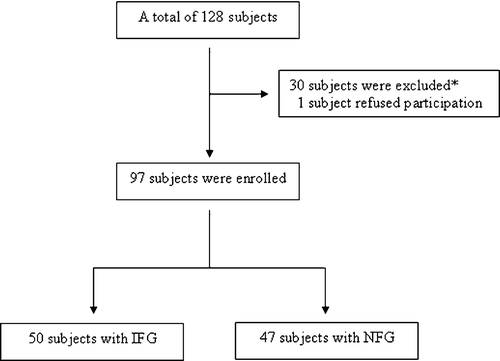
This study is a single‐center, open‐label, and non‐randomized study. A total of 128 asymptomatic volunteer subjects were recruited for the study – 120 patients with exercise capacity underwent treadmill testing with Bruce protocol and the remaining eight patients unable to exercise underwent a pharmacological scintigraphic stress test. Three subjects with abnormal test results were excluded from the study. All patients underwent selective coronary angiography and during procedure measurements of ascending aortic systolic and diastolic pressures were performed. The values obtained were used for calculation of mean pressure, PP and FPPs. An additional 24 patients were excluded from the study after the angiographic detection of coronary atherosclerotic lesion. Three subjects were also excluded because of the detection of coronary slow flow (CSF, described in the section of cardiac catheterization). In addition, one patient refused participation in the study. A fasting peripheral venous blood sample from all patients was obtained before entering the study. Patients having glucose level of 100–125 mg/dl after at least a 12‐h fasting state were enrolled in the IFG group. On the other hand, patients having glucose values <100 mg/dl were enrolled in the normal fasting glucose (NFG) group. The remaining 97 patients were enrolled and divided into two groups according to fasting plasma glucose (FPG). In the first group, 50 subjects (18 men, 32 women; mean age 56.8±12.2 years) with IFG, in the second group 47 subjects (20 men, 27 women; mean age 53.1±11.2 years) with NFG were present (Figure ).
Figure 1 Study design. The disposition of the participants.*Three patients were excluded after abnormal stress testing, 24 patients were excluded after coronary angiography with atherosclerotic lesions. Additionally three patients with CSF were excluded from the study. IFG, impaired fasting glucose; NFG, normal fasting glucose.
None of the patients was on drug therapy at the time of admission to hospital. During two consecutive weekly clinic visits, blood pressure and FPG measurements were obtained for all patients. Hypertension was diagnosed if the average of the three blood pressure measurements at the two clinic visits was consistently elevated over 140 systolic and/or 90 diastolic. In addition, previously diagnosed and treated hypertensive states were accepted as hypertension, although measured blood pressures were lower than 140 systolic and/or 90 diastolic. Glucose level of 100–125 mg/dl after at least a 12‐h fasting state was accepted as IFG. Values <100 mg/dl were within normal limits and accepted as NFG. Exclusion criteria included the following: known coronary artery disease; left ventricular dysfunction (left ventricular ejection fraction <50%) and hypertrophy; unstable ischemic conditions (unstable angina pectoris and myocardial infarction); valvular heart disease; rhythms other than sinus; metabolic syndrome; and detection of coronary atherosclerotic lesion and/or CSF after selective coronary artery angiography. Lastly, an inguinal hematoma at the access site occurred in one subject, and during cardiac catheterization hypotension with bradycardia was observed in another patient as procedural complications. The Institutional Ethics Committee approved the study protocol and all patients gave informed consent to participate in the study.
Cardiac catheterization
All patients in the study underwent selective coronary artery angiography after appropriate patient preparation. Femoral artery cannulation was used for the arterial access site and a Judkins system was applied for cannulating the left and right coronary arteries. All angiograms were evaluated by two experienced physicians blinded to the study. Angiograms without stenotic lesion in all major epicardial coronary arteries including left main (LM), left anterior descending (LAD), left circumflex (LCX) and right coronary arteries (RCAs) were considered normal angiograms. In addition, CSF was investigated by using thrombosis in myocardial infarction (TIMI) frame count method described by Gibson et al. Citation[8]. Previously published normal TIMI frame counts were 21.1±1.5 frames for LAD (after correction), 22.2±4.1 frames for LCX and 20.4±3.0 frames for RCA Citation[8]. For a given artery, any value above this published range was considered CSF and accepted as an exclusion criterion.
Measurement of hemodynamic parameters
Hemodynamic measurements including systolic and diastolic blood pressures were assessed using the pigtail system during cardiac catheterization for each patient. The average of at least five pressure waveforms on the paper with a speed of 25 mm/s were used for analysis by a physician blinded to the study.
Calculation of hemodynamic parameters:
Aortic PP = aortic systolic pressure−aortic diastolic pressure;
Aortic mean pressure = 1/3 aortic systolic pressure+2/3 aortic diastolic pressure;
Aortic FPP = aortic PP/aortic mean pressure.
Laboratory data
Fasting peripheral venous blood samples were obtained from all patients in the study for the measurement of FPG, total cholesterol, low‐density lipoprotein (LDL)‐cholesterol, high‐density lipoprotein (HDL)‐cholesterol and triglyceride levels. Blood samples were centrifuged and plasma was obtained. FPG, total cholesterol, HDL‐ cholesterol and triglyceride levels were measured by different laboratory techniques. Plasma glucose was measured with the glucose oxidase technique. Measurement of LDL‐cholesterol level was done through application of a formula as described by Friedewald et al. Citation[9].
Anthropometric measurement
Height and weight of patients were measured and body mass index (BMI) was calculated by dividing weight in kilograms by height in meters squared, and described as kg/m2.
Statistical analysis
Data were analyzed with the SPSS software version 15.0 for Windows (SPSS Inc., Chicago, Illinois, USA). Continuous variables were presented as mean±SD and categorical variables as frequency and percentage. Student's t‐test was used to compare normally distributed continuous variables and the Mann–Whitney U test for variables without normal distribution. The χ2 test was used to compare categorical variables. Any correlation between aortic FPP and FPG was analyzed by the linear regression model. A two‐tailed p‐value of <0.05 was considered statistically significant.
Results
Baseline characteristics
Baseline demographic and laboratory characteristics of patients in both groups were outlined in Table . Thirty‐six per cent of patients were male with a mean age of 52.2±13.5 years, and 64% of the cases were female with a mean age of 59.4±10.7 years in the IFG group; 43% of patients were male and 57% were female with a mean age of 51.6±13.8 and 54.2±9.0 years in NFG group, respectively. The mean FPG level was significantly higher in IFG group compared with controls (p<0.001). There were no significant differences between the two groups concerning age, sex and other cardiovascular risk factors, including hypertension and smoking. However, the prevalence of hypertension tended to be higher in the study group (p = 0.06). In addition, both groups did not significantly differ in BMI, total, HDL‐, LDL‐cholesterols and triglyceride levels.
Table I. Baseline demographic and laboratory characteristics.
Hemodynamic parameters
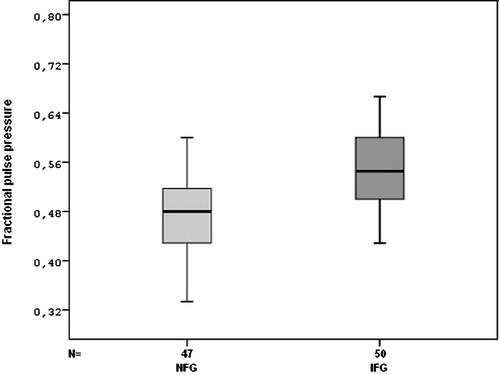
Heart rate measured during catheterization was not different between two groups. However, measured aortic systolic, diastolic, mean pressure, PP and FPPs were significantly higher in patients with IFG than in those with NFG (Table , Figure ).
Table II. Hemodynamic parameters.
Figure 2 Comparison of impaired fasting glucose(IFG) and normal fasting glucose (NFG) groups for fractional pulse pressure (FPP).
There was no significant gender influence on the aortic PP and FPPs–IFG relationship in our study: women, 56±15 mmHg; men, 51±8 mmHg, p = 0.131 for aortic PP; women, 0.57±0.12; men, 0.55±0.06, p = 0.435 for aortic FPP.
In addition, mean FPG had significant positive correlation with BMI, aortic systolic, diastolic, mean pressure and PPs (Table ). No significant correlation was detected between mean FPG and serum total‐, LDL‐, HDL‐cholesterol, triglyceride or heart rate measurements (Table ).
Table III. Correlation between mean FPG and anthropometric, aortic blood pressure, and laboratory measurements.
According to BMI, in the NFG group and the IFG group, there were no significant correlations between BMI and the aortic PP/FPPs.
In the NFG group, R = 0.026 and p = 0.863 for BMI and PP; R = 0.085 and p = 0.568 for BMI and FPP.
In the IFG group, R = 0.195 and p = 0.175 for BMI and PP; R = 0.055 and p = 0.703 for BMI and FPP.
There were also no significant correlations between age and the aortic PP/FPPs (p = 0.099 and 0.573, respectively).
Lastly, a significant positive correlation between FPG levels and measured aortic FPPs was found by linear regression analysis (p = 0.001, R2 = 0.12, β = 0.347) (Figure ).
Discussion
In our study, we found that patients with IFG had significantly increased aortic PP and FPPs compared with control subjects.
Increased PP has been shown to be independently associated with adverse cardiovascular events in large cohorts of the population Citation[2], Citation[3]. Although these studies have included male subjects largely, it has been shown that increased aortic PP and FPPs are significantly associated with the presence of angiographic coronary artery disease in women Citation[6]. Although measured brachial PP has been shown to be significantly associated with the risk of coronary heart disease Citation[3], invasively measured aortic PP and evaluated FPP via catheterization gives more accurate and reliable values compared with sphygmomanometer‐measured brachial blood pressure. PP also increases from central to peripheral arteries. The reliability of derived aortic BP is crucially dependent on the validity of the transfer function used to generate the central aortic waveforms. Increased aortic PP is associated with increased aortic stiffness. In addition, variations in PP are commonly a consequence of variations in the mean pressure. Increased PP may accordingly be due to distension of the aortic wall, without changes in the wall structure. It may be possible to correct for this by calculating the FPP. Therefore, accurate FPP measures aortic stiffness. Atherosclerosis produces many pathological changes in the arterial wall, one of the most important being a progressive increase in arterial stiffening, although hypertension may also increase aortic stiffness. When the aortic wall has stiffened due to atherosclerotic process, systolic blood pressure increases and the diastolic blood pressure decreases relative to mean pressure because of shortened time constant of diastolic blood pressure decay resulted in increased aortic PP. Therefore, central aortic PP is a more reliable and accurate indicator of aortic stiffness. Aortic FPP has also been shown to be strongly associated with angiographic coronary heart disease in men and women Citation[5], Citation[6]. Because aortic stiffness has been shown to be related to coronary atherosclerosis Citation[10], aortic PP is more strongly associated with coronary atherosclerosis than the peripheral PP. In addition, other than aortic PP, it has been also shown that FPP is independently associated with coronary atherosclerosis Citation[5], Citation[6]. In the present study, we studied patients with normal coronary angiograms. Therefore, which mechanism is responsible for the significant increase in aortic PP and FPPs in patients with IFG compared with control subjects? The endothelium of the vascular tissue has many functions for the continuation of the normal vascular physiology. It plays a key role in maintaining vascular homeostasis through balancing endothelial‐derived relaxing and contracting factors. The role of the endothelium in controlling the vascular tone, especially vasodilatation, has been shown via the endothelial‐derived relaxing factor, which was later named as NO Citation[11], Citation[12]. Endothelial dysfunction occurs when NO activity in the vascular tissue is decreased, resulting in decreased endothelium‐dependent vasodilatation. McEniery et al. Citation[13] showed that endothelial function was inversely associated with aortic pulse wave velocity and augmentation index in a large cohort of healthy volunteers. In their study, both endothelium‐dependent and endothelium‐independent vasodilatation was evaluated through the change in augmentation index. This association was also more prominent with central PP compared with brachial PP Citation[13]. IFG may be causally related to coronary vascular dysfunction. Hyperglycemia, even below the threshold of diabetes, is also associated with impaired endothelial function, and subjects with impaired glucose metabolism, without overt diabetes, have increased risk of cardiovascular disease Citation[14–16]. Relatively small increases in fasting and post‐prandial glucose levels (including IFG or impaired glucose tolerance) confer an increased risk for cardiovascular morbidity and mortality. Potential mechanisms underlying the association between elevated glucose concentrations in the non‐diabetic range and atherosclerosis include oxidative stress, LDL oxidation, haemostatic factors, reduced NO production, advanced glycation endproduct formation on the vessel walls, increased endothelin 1, inflammatory factors and vasoconstrictive prostanoids Citation[17], Citation[18]. When plasma and cell membrane proteins are exposed to chronic high glucose concentrations, they undergo non‐enzymatic glycosylation and cross‐linking; the resultant advanced glycosylation endproducts can inhibit NO Citation[19]. Patients with impaired glucose metabolism display a number of features including negative effects on smooth muscle cell relaxation by advanced glycation endproducts, and in some cases diabetic neuropathy, but the major common pathway seems to be endothelial dysfunction Citation[20]. Therefore, higher aortic PP and FPPs in patients with IFG might be the result of endothelial dysfunction. In addition, autonomic imbalance in the central and especially in peripheral nervous system, which is another underlying pathophysiological mechanism in IFG, might cause increased aortic PP and FPPs in this clinical entity. Sympathetic nervous hyperactivity results in increased heart rate and systemic vascular resistance. Thus elevated sympathetic tonus causes increased systolic and unchanged or decreased diastolic blood pressures. Therefore, aortic PP and so FPP are increased.
Study limitations
The major limitation of this study is the limited sample size, which means that the results have to be confirmed in a larger study. In addition, this is a clinical study. Therefore, at the molecular level, endothelial function/dysfunction might be evaluated and results might be combined with clinical factors. Lastly, the use of a Millar conductance catheter for aortic pressure measurement is more appropriate than the use of a pigtail system catheter.
Conclusion
We have demonstrated that aortic PP and FPPs, indicators of the risk of coronary artery disease, in patients with IFG, was significantly elevated compared with control subjects with NFG. Prospective studies with large sample sizes are needed for prognostic evaluation of increased aortic PP and FPPs in patients with IFG in real‐life practice on long‐term follow‐up.
Acknowledgement
There are no conflicts of interest regarding the authors.
References
- Coutinho M., Gerstein H. C., Wang Y., Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care 1999; 22: 233–40
- Benetos A., Safar M., Rudnichi A., Smulyan H., Richard J‐L. Pulse pressure: A predictor of longterm cardiovascular mortality in a French male population. Hypertension 1997; 30: 1410–5
- Franklin S. S., Khan S. A., Wong N. D., Larson M. G., Levy D. Is pulse pressure useful in predicting risk for coronary heart disease? The Framingham Heart Study. Circulation 1999; 100: 354–60
- Jankowski P., Kawecka‐Jaszcz K., Czarnecka D., Brzozowska‐Kiszka M., Styczkiewicz K., Styczkiewicz M., et al. Ascending aortic, but not brachial blood pressure‐derived indices are related to coronary atherosclerosis. Atherosclerosis 2004; 176: 151–5
- Nishijima T., Nakayama Y., Tsumura K., Yamashita N., Yoshimaru K., Ueda H., et al. Pulsatility of ascending aortic blood pressure waveform is associated with an increased risk of coronary heart disease. Am J Hypertens 2001; 14: 469–73
- Guray Y., Guray U., Altay H., Cay S., Yilmaz M. B., Kisacik H. L., et al. Aortic pulse pressure and aortic pulsatility are associated with angiographic coronary artery disease in women. Blood Press 2005; 14: 293–7
- Wakabayashi I., Masuda H. Association of pulse pressure with carotid atherosclerosis in patients with type 2 diabetes mellitus. Blood Press 2007; 16: 56–62
- Gibson C. M., Cannon C. P., Daley W. L., Dodge J. T Jr., Alexander B Jr., Marble S. J., et al. TIMI frame count: A quantitative method of assessing coronary artery flow. Circulation 1996; 93: 879–88
- Friedewald W. T., Levy R. I., Fredrickson D. S. Estimation of the concentration of low density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18: 499–502
- Weber T., Auer J., O'Rourke M. F., Kvas E., Lassnig E., Berent R., et al. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation 2004; 109: 184–9
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980; 288: 373–6
- Moncada S., Higgs A. The L‐arginine‐nitric oxide pathway. N Engl J Med 1993; 329: 2002–12
- McEniery C. M., Wallace S., Mackenzie I. S., McDonnell B., Yasmin., Newby D. E., et al. Endothelial function is associated with pulse pressure, pulse wave velocity, and augmentation index in healthy humans. Hypertension 2006; 48: 602–8
- Binak E., Gunduz H., Sahin M., Kurtoglu N., Dindar I. The relation between impaired glucose tolerance and slow coronary flow. Int J Cardiol 2006; 111: 142–6
- Caballero A. E., Arora S., Saouaf R., Lim S. C., Smakowski P., Park J. Y., et al. Microvascular and macrovascular reactivity is reduced in subjects at risk for type 2 diabetes. Diabetes 1999; 48: 1856–62
- Srinivasan M., Herrero P., McGill J. B., Bennik J., Heere B., Lesniak D., et al. The effects of plasma insulin and glucose on myocardial blood flow in patients with type 1 diabetes mellitus. J Am Coll Cardiol 2005; 46: 42–8
- Tesfamariam B., Brown M. L., Deykin D., Cohen R. A. Elevated glucose promotes generation of endothelium‐derived vasoconstrictor prostanoids in rabbit aorta. J Clin Invest 1990; 85: 929–32
- Fuller J. H., Shipley M. J., Rose G., Jarrett R. J., Keen H. Coronary heart disease risk and impaired glucose tolerance: The Whitehall study. Lancet 1980; 1: 1373–6
- Bucala R., Tracey K. J., Cerami A. Advanced glycosylation products quench nitric oxide and mediate defective endothelium‐dependent vasodilatation in experimental diabetes. J Clin Invest 1991; 87: 432–8
- De Angelis L., Marfella M. A., Siniscalchi M., Marino L., Nappo F., Giugliano F., et al. Erectile and endothelial dysfunction in Type II diabetes: A possible link. Diabetologia 2001; 44: 1155–60