Abstract
Background. The role of endogenous relaxin on hypertensive cardiovascular damage remains unknown. We investigated the relaxin level and its relation to cardiovascular function in patients with never treated hypertension (HT). Methods. We studied 42 (47.8±10 years) never treated patients with HT and 40 age‐matched (47±8.6 years) normotensive individuals. Serum relaxin levels were determined in all subjects using enzyme‐linked immunosorbent assay. Left ventricular (LV) diameters were evaluated by transthoracic echocardiography. Ejection fraction and LV mass index were measured. Diastolic functions were evaluated with both conventional and tissue Doppler echocardiography. We evaluated central aortic pressures, heart rate‐corrected augmentation index (AIx@75), a marker of wave reflections, and aortic pulse wave velocity (PWV) as indices of elastic‐type aortic stiffness of the study population using applanation tonometry (SphygmoCor). Results. Relaxin levels were significantly lower in hypertensive patients as compared with controls (36.5±7.3 vs 49.7±39.8 pg/ml, p=0.03). The relaxin level was negatively correlated with brachial and central aortic pressure. However, serum relaxin was not significantly associated with LV diameters, ejection fraction, LV mass index, LV diastolic function, AIx@75 or aortic PWV in our study. Conclusion. Serum relaxin is decreased in patients with HT. However, low endogenous relaxin is not related to cardiovascular function.
Introduction
Relaxin is a 6‐kD polypeptide hormone that belongs to the insulin family Citation[1]. In women, relaxin is produced by the corpus luteum, both in the luteal phase of the menstrual cycle and throughout pregnancy, whereas in men, relaxin is secreted by the prostate gland and appears in seminal plasma Citation[1]. Recent studies provide evidence that relaxin may contribute to cardiovascular adaptations during pregnancy through effects on the kidney, vasculature and heart Citation[2]. Recent evidence from experimental studies using exogenous relaxin in rats show that the peptide may also play a role in the cardiovascular system owing to its vasodilatory, diuretic and central hemodynamic effects Citation[3–5]. It has been suggested that there is a potential role of relaxin in preeclampsia Citation[6].
Hypertensive patients commonly have demonstrated fibrosis and extracellular matrix remodeling that predispose to hypertensive heart disease Citation[7]. Relaxin has been shown to limit the influence of profibrotic factors Citation[1–5] and is negatively associated with ventricular diastolic function, left ventricular (LV) hypertrophy and systemic arterial resistance Citation[8–10]. The cardiovascular effects of relaxin could potentially also counteract some pathophysiological mechanisms resulting in target organ damage in patients with HT. However, the exact role of endogenous relaxin on hypertensive cardiovascular damage remains unknown. In this study, we investigated circulating relaxin levels and its relation to cardiovascular function in newly diagnosed never treated hypertensive patients.
Methods
Study population
In this study, 42 consecutive newly diagnosed hypertensive individuals (47.8±10 years) were enrolled. Forty age‐ and sex‐matched normal subjects (47±8.6 years) were also studied. The diagnosis of HT was established according to the JNC seventh report Citation[11]. Pulse wave analysis and velocity were evaluated using applanation tonometry (SphygmoCor). Two‐dimensional echocardiographic examinations were performed on each subject. Subjects with HT had not previously taken any antihypertensive therapy. Patients with hemolytic, hepatic and renal diseases, diabetes mellitus, heart failure, valvular heart disease, ejection fraction less than 50%, history of coronary artery disease or acute coronary syndromes, pregnancy, hypertrophic cardiomyopathy were excluded from the study. Written informed consent was obtained from each subject, and the institutional ethics committee of KTU approved the study protocol.
Echocardiographic examination
The echocardiographic examinations were obtained by using GE VingMed System 7 (Norway). Two‐dimensional, M‐mode, and subsequent standard and pulsed tissue Doppler echocardiographic examinations were performed on each subject in the lateral decubitus position. The LV end‐systolic, end‐diastolic diameters, end‐diastolic interventricular septal thickness, end‐diastolic posterior wall thickness and left atrial diameter were measured by using the American Echocardiography Society M‐mode technique Citation[12]. LV ejection fraction was determined by method of Teichholz et al. Citation[13]. Mitral flow diastolic E and A wave velocity, E wave deceleration time and LV isovolumetric relaxation time, between end point of aortic wave trace and beginning of mitral wave trace, were measured by locating the sampling volume cursor of pulsed Doppler in the apical four‐chamber position at 5 mm above the end point of mitral leaflets Citation[14]. Then, adjusting the equipment of echocardiography to tissue Doppler measurements, the sampling volume cursor was located at the cross‐sectional point of mitral annulus and lateral wall. Using the traces obtained from this location, mitral annular systolic wave velocities, early (Em) and late (Am) diastolic tissue Doppler velocities were measured Citation[15]. LV mass was calculated from M‐mode records taken on parasternal long‐axis images according to the formula below (corrected American Society of Echocardiography cube method) Citation[12],Citation[16]. To take into account differences in body size that might influence cardiac size, LV mass was divided by height to create an LV mass index.
LV mass=0.8×(1.04[(IVSd+PWd+LVDD)3(LVDD)3])+0.6g
Measurement of relaxin
Blood samples of for measuring relaxin levels was drawn from an antecubital vein. Serum was immediately obtained by centrifugation of the blood at 3000g for 10 min at 4°C and then stored in several aliquots at −20°C until assayed. Relaxin levels were analyzed with the use of a commercial enzyme‐linked immunosorbent assay (ELISA) kits (Immundiagostik GmbH, Bensheim, Germany), according to the manufacturer's instructions. The minimal detectable concentration for the relaxin assay was 0.40 pg/ml. The intra‐assay coefficient of variation is 9.6% and the interassay coefficient of variation is 10.2%. Cross‐reactivity against insulin, insulin‐like growth factors, LH, FSH and prolactin is less than 0.01%.
Blood pressure measurement
Brachial artery blood pressure was measured with a mercury sphygmomanometer in an office setting; the first and fifth phases of Korotkoff sounds were used for systolic and diastolic blood pressure. Appropriate cuff sizes were chosen for each subject's arm circumference. In each subject, brachial artery blood pressure was measured on at least three separate days after 15 min of comfortably sitting and the average of the measurements was recorded. According to guidelines from the JNC 7 report, HT was defined as a systolic BP of ≥140 mmHg or diastolic BP of ≥90 mmHg Citation[11].
Measurement of pulse wave velocity
Aortic PWV was determined with the foot‐to‐foot method using the SphygmoCor system (AtCor Medical, Sydney, Australia) Citation[17]. Consecutive registrations of the carotid and femoral artery pulse waves are electrocardiogram gated and thus, the time shift between the appearance of wave at the first and the second sites can be calculated. The distance between the two sites was measured on the body surface; to determine aortic PWV in meters/second (m/s). We used the total distance between the carotid and femoral sites of measurement. The average of measurements over a period of 8 s Citation[9–10] cardiac cycles) was calculated after the exclusion of extreme values.
Pressure waveform analysis
Assessment of arterial wall properties and wave reflection characteristics was performed non‐invasively using the SphygmoCor system. Radial artery pressure waveforms were recorded at the wrist, using applanation tonometry with a high‐fidelity micromanometer (Millar Instruments, Houston, Texas). After 20 sequential waveforms had been acquired and averaged, a validated generalized mathematical transfer function was used to synthesize the corresponding central aortic pressure waveform Citation[18]. The augmentation index (AIx) and augmentation pressure (AP) were derived from this with the technique of pressure waveform analysis. The merging point of the incident and the reflected wave (the inflection point) was identified on the generated aortic pressure waveform. AP was the maximum systolic pressure minus pressure at the inflection point. The AIx was defined as the AP divided by pulse pressure and expressed as a percentage. Larger values of AIx indicate increased wave reflection from the periphery or earlier return of the reflected wave as a result of increased pulse wave velocity (attributable to increased arterial stiffness). AIx is dependent upon the elastic properties of the entire arterial tree (elastic and muscular arteries). In addition, because AIx is influenced by heart rate, an index normalized for heart rate of 75 beats/min (AIx@75) was used in accordance with Wilkinson et al. Citation[19].
Only high‐quality recordings, defined as an in‐device quality index of >80% (derived from an algorithm including average pulse height, pulse height variation, diastolic variation and the maximum rate of rise of the peripheral waveform) and acceptable curves on visual inspection, were included in the analysis. All measurements were performed by the same person (O. Gedikli) with the patient in the supine position in a quiet temperature‐controlled room after a brief rest period of at least 5 min.
Statistical analyses
Continuous data are expressed as the mean±SD. Comparison between two groups was performed using the unpaired t‐test or non‐parametric means test (Mann–Whitney U test) for continuous variables, and using the Fisher exact test for categorical variables. Associations between serum relaxin levels and other variables were evaluated by the Pearson correlation test. A p‐value of <0.05 was considered statistically significant. Statistical analyses were performed using SPSS software (Version 10.0, SPSS, Inc., Chicago, IL).
Results
Patients' characteristics
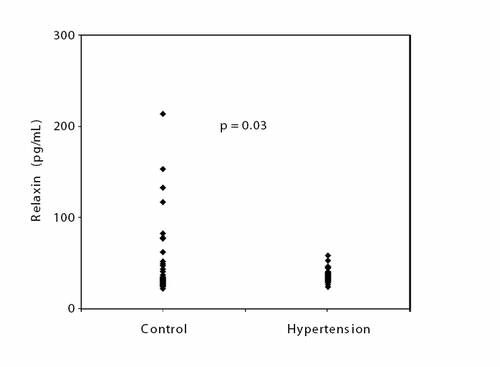
Baseline clinical and demographic characteristics of the study population are shown in . There were no significant differences in age, gender, smoking, heart rate, body mass index, fasting glucose, hemoglobin, serum creatinine and lipid profiles between the groups. Serum relaxin level was significantly lower in patients with HT than control (36.5±7.3 vs 49.7±39.8 pg/ml,p=0.03) (). Relaxin level was similar between male and female subjects (45±34 vs 39±14,p=0.34).
Table I. Clinical and biochemical characteristics of the study groups.
Pulse wave analysis and velocity
The indices of arterial stiffness and wave reflections of the study population are presented in . Central aortic systolic, diastolic and pulse pressure were significantly higher in patients with HT than control subjects. Augmentation pressure, AIx, AIx@75 and aortic PWV were significantly higher in patients with HT than control subjects.
Table II. Pulse wave analysis and velocity in the study groups.
Baseline echocardiographic characteristics
The echocardiographic data of the groups are shown in . There were no significant differences in LV diameters and ejection fraction between the groups. Patients with HT had a greater LV wall thickness, left atrial diameter and LV mass index than control. HT group had a significantly lower mitral flow diastolic E wave velocity and E/A ratio than the control subjects. Also, mitral flow diastolic A wave velocity, isovolumetric relaxation time and late diastolic mitral annular velocity were significantly higher in patients with HT than in controls.
Table III. Echocardiographic measurements of study population.
Relationship between serum relaxin level and cardiovascular function
In bivariate correlation analysis, negative significant correlation was found between serum relaxin level and brachial systolic pressure (r= −0.28,p=0.011), brachial diastolic pressure (r= −0.27,p=0.013), central aortic systolic pressure (r= −0.24,p=0.03) and central aortic diastolic pressure (r= −0.26,p=0.015). However, serum relaxin level was not associated with brachial pulse pressure (r= −0.18,p=0.1) and central aortic pulse pressure (r= −0.13,p=0.2). No significant relationships were observed between relaxin and LV diameters, ejection fraction, LV mass index, LV diastolic function, AIx@75 and aortic PWV.
Discussion
In the present study, we investigated the relaxin level and its relation to cardiovascular function in patients with HT. We have found that serum relaxin level was significantly lower in patients with HT compared with normotensive subjects. Also, relaxin level was related to brachial and central aortic pressure. However, serum relaxin level was not associated with LV diameters, ejection fraction, LV mass index, LV diastolic function, AIx@75 and aortic PWV in our study. Previous studies show that pulse pressure is major determinant of cardiovascular risk Citation[20],Citation[21]. In our study, serum relaxin level was not associated with brachial and aortic pulse pressure.
Relaxin has many important roles in pregnancy, including softening effects on connective tissue, reducing uterine contractility, and control of mammary gland growth and differentiation Citation[3]. Recently, however, it was recognized that relaxin also plays a role in the cardiovascular system Citation[3–5]. Therefore, the role of relaxin on the pathophysiology of cardiovascular disease may potentially have an interest for developing new treatment strategies.
Impaired LV diastolic function and increased LV mass are now recognized as common findings in hypertensive patients Citation[22]. Dschietzig et al. Citation[23] showed that endogenous myocardial relaxin upregulated in left heart hypertrophy and inversely correlated with the degree of hypertrophy in male spontaneously hypertensive rats. Du et al. Citation[8] found that male relaxin deficient mice had increased LV diastolic filling and, most likely related to an increase in ventricular collagen content and chamber stiffness. These changes were not observed in female relaxin deficient mice. On the other hand, Xu et al. Citation[24] demonstrated that, although upregulated in relaxin MRNA, endogenous relaxin had no significant effect on the LV hypertrophy, fibrosis and dysfunction in the setting of chronic pressure overload. In our study, serum relaxin level was not associated LV mass index and LV diastolic function in correlation analysis.
In our study, relaxin level negatively related to brachial and aortic pressure. However, some animal studies show that relaxin does not affect blood pressure Citation[25],Citation[26]. It is conceivable that endogenous circulating relaxin contributes to decline in blood pressure. Previous studies have shown that aortic compliance decreases in hypertensive individuals Citation[27]. Compared with normotensive individuals, aortic PWV and AIx@75 were higher in our hypertensive subjects, indicating a deterioration in arterial stiffness and wave reflections. That is, arterial stiffness itself is a complex phenomenon consisting of several distinct processes, which include structural elements within the arterial wall, vascular smooth muscle tone and impaired endothelial function Citation[27]. In our study, relaxin level was not related to arterial function indices. Debrah et al. Citation[10] found that chronic administration of recombinant human relaxin reduces systemic vascular resistance in hypertensive rats. It was shown that relaxin is a vasodilator of small systemic resistance arteries Citation[2]. However, relaxin does not promote vasodilation in all blood vessels Citation[28]. Fisher et al. Citation[28] found that relaxin‐induced vasodilation is endothelium dependent. Removal of the endothelium almost abolished its effect Citation[28]. On the other hand Failli et al. Citation[29] reported that relaxin markedly reduced the [Ca2+]i response of vascular smooth muscle and endothelial cells from normotensive rats, but not from spontaneously hypertensive rats. In that study, it was found that vascular smooth muscle and endothelial cells in spontaneously hypertensive rats showed a deficiency response to nitric oxide that may render them insensitive to relaxin Citation[29].
In a previous study, Dschietzig et al. Citation[30] showed that relaxin is constitutively expressed in cardiovascular tissues in patients with heart failure. Also plasma levels of relaxin in patients increase profoundly with the severity of heart failure Citation[30]. Kupari et al. Citation[31] found that although the heart may release relaxin into the circulation in heart failure, systemic concentration of relaxin was not elevated. Furthermore, the relaxin level did not correlate with cardiac structure and function Citation[31]. Kruger et al. Citation[32] reported that relaxin concentrations at rest and after exercise were similar in patients with heart failure and in controls. Fisher et al. Citation[33] demonstrated that relaxin did not predict prognosis in patients with chronic heart failure. Although relaxin levels were increased in patients with heart failure, relaxin was not associated with pathophysiological alterations. Similarly, we found that serum relaxin level was not related to hypertensive cardiovascular damage.
Study limitations
The small number of patients is a potential limitation of this study. Larger studies are needed to establish the potential role of relaxin on pathophysiological cardiovascular changes in patients with HT.
In conclusion, serum relaxin level was decreased in patients with HT. However, the serum relaxin level was not related to hypertensive cardiovascular damage.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bryant‐Greenwood GD, Schwabe C. Human relaxins: Chemistry and biology. Endocr Rev 1994; 15: 5–26
- Sherwood OD. Relaxin's physiological roles and other diverse actions. Endocr Rev 2004; 25: 205–234
- Bani D. Relaxin: a pleiotropic hormone. Gen Pharmacol 1997; 28: 13–22
- Geddes BJ, Summerlee AJS. The emerging concept of relaxin as a centrally acting peptide hormone with hemodynamic actions. J Neuroendocrinol 1995; 7: 411–417
- Conrad KP, Novak J. Emerging role of relaxin in renal and cardiovascular function. Am J Physiol Regul Integr Comp Physiol 2004; 287: R250–R261
- Mohaupt M. Molecular aspects of preeclampsia. Mol Aspects Med 2007; 28: 169–191
- Weber KT. Fibrosis and hypertensive heart disease. Curr Opin Cardiol 2000; 15: 264–272
- Du XJ, Samuel CS, Gao XM, Zhao L, Parry LJ, Tregear GW. Increased myocardial collagen and ventricular diastolic dysfunction in relaxin deficient mice: a gender‐specific phenotype. Cardiovasc Res 2003; 57: 395–404
- Lekgabe ED, Kiriazis H, Zhao C, Xu Q, Moore XL, Su Y, et al. Relaxin reverses cardiac and renal fibrosis in spontaneously hypertensive rats. Hypertension 2005; 46: 412–418
- Debrah DO, Conrad KP, Jeyabalan A, Danielson LA, Shroff SG. Relaxin increases cardiac output and reduces systemic arterial load in hypertensive rats. Hypertension 2005; 46: 745–750
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA 2003; 289: 2560–2572
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. American Society of Echocardiography's Nomenclature and Standards Committee; Task Force on Chamber Quantification; American College of Cardiology Echocardiography Committee; American Heart Association; European Association of Echocardiography, European Society of Cardiology. Recommendations for chamber quantification. Eur J Echocardiogr 2006; 7: 79–108
- Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: Echocardiographic‐ angiographic correlations in the presence of absence of asynergy. Am J Cardiol 1976; 37: 7–11
- Cohen GI, Pietrolungo JF, Thomas JD, Klein AL. A practical guide to assessment of ventricular diastolic function using Doppler echocardiography. J Am Coll Cardiol 1996; 27: 1753–1760
- Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol 1997; 30: 1527–1533
- Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 1986; 57: 450–458
- Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006; 27: 2588–2605
- Pauca AL, O′Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension 2001; 38: 932–937
- Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol 2000; 525: 263–270
- Blacher J, Staessen JA, Girerd X, Gasowski J, Thijs L, Liu L, et al. Pulse pressure not mean pressure determines cardiovascular risk in older hypertensive patients. Arch Intern Med 2000; 160: 1085–1089
- Benetos A, Safar M, Rudnichi A, Smulyan H, Richard JL, Ducimetieère P, et al. Pulse pressure: a predictor of long-term cardiovascular mortality in a French male population. Hypertension 1997; 30: 1410–1415
- Diamond JA, Phillips RA. Hypertensive heart disease. Hypertens Res 2005; 28: 191–202
- Dschietzig T, Bartsch C, Kinkel T, Baumann G, Stangl K. Myocardial relaxin counteracts hypertrophy in hypertensive rats. Ann NY Acad Sci 2005; 1041: 441–443
- Xu Q, Lekgabe ED, Gao XM, Ming Z, Tregear GW, Dart AM, et al. Endogenous relaxin does not affect chronic pressure overload-induced cardiac hypertrophy and fibrosis. Endocrinology 2008; 149: 476–482
- Parry LJ, Poterski RS, Summerlee AJS. Effects of relaxin on blood pressure and the release of vasopressin and oxytocin in anesthetized rats during pregnancy and lactation. Biol Reprod 1994; 50: 622–628
- Ward DG, Cronin MJ, Baertschi AJ. Lack of cardiovascular and vasopressin responses to human relaxin in conscious, late pregnant rats. Am J Physiol 1991; 261: 206–211
- Laurent S, Boutouyrie P. Recent advances in arterial stiffness and wave reflection in human hypertension. Hypertension 2007; 49: 1202–1206
- Fisher C, MacLean M, Morecroft I, Seed A, Johnston F, Hillier C, et al. Is the pregnancy hormone relaxin also a vasodilator peptide secreted by the heart?. Circulation 2002; 106: 292–295
- Failli P, Nistri S, Mazzetti L, Chiappini L, Bani D. Effects of relaxin on vascular smooth muscle and endothelial cells in normotensive and hypertensive rats. Ann N Y Acad Sci 2005; 1041: 311–313
- Dschietzig T, Richter C, Bartsch C, Laule M, Armbruster FP, Baumann G, et al. The pregnancy hormone relaxin is a player in human heart failure. FASEB J 2001; 15: 2187–2195
- Kupari M, Mikkola TS, Turto H, Lommi J. Is the pregnancy hormone relaxin an important player in human heart failure?. Eur J Heart Fail 2005; 7: 195–198
- Krüger S, Graf J, Merx MW, Stickel T, Kunz D, Hanrath P, et al. Relaxin kinetics during dynamic exercise in patients with chronic heart failure. Eur J Intern Med 2004; 15: 54–56
- Fisher C, Berry C, Blue L, Morton JJ, McMurray J. N‐terminal pro B type natriuretic peptide, but not the new putative cardiac hormone relaxin, predicts prognosis in patients with chronic heart failure. Heart 2003; 89: 879–881