Abstract
Background: The efficacy and safety of renin angiotensin aldosterone system blockers (RAASB’s) if introduced immediately after renal transplantation have not been extensively investigated.
Methods: The medical charts of 142 kidney transplant recipients who received a RAASB in the early postoperative period and of 114 matched controls were analyzed. The RAASB was given primarily for blood pressure control.
Results: 117 patients continued to receive and 50 controls remained continuously free of the RAASB in the first year. The RAASB was added on average at postoperative day 8 and the mean duration of follow-up was 5.4 years. Systolic, blood pressure at treatment initiation was increased in the RAASB group (150 ± 17 vs. 141 ± 16, p < 0.001). At discharge from hospital and during follow-up blood pressure was similar in both groups, without differences in GFR, potassium and proteinuria. The endpoints “graft failure” and “graft failure or death from any cause” were significantly better in patients treated with RAASB’s (p = 0.03 and p = 0.04, respectively). The treatment effects in the RAASB group persisted even after adjustment for demographic parameters, immunological risk factors, peritransplant risk factors, duration of dialysis prior to transplantation and medical comorbidities.
Conclusions: Thus, RAASB’s can be used effectively and safely to treat hypertension in the early postoperative period after kidney transplantation and are renoprotective in the long term.
Introduction
Blood pressure control is an important variable in patient care and presents one of the major influential risk factors to decrease cardiovascular risk and improve allograft survival in renal transplant recipients.[Citation1,Citation2] The use of ACEI’s/ARB’s after renal transplantation is still limited [Citation3] despite their well-known blood pressure independent cardio- and reno-protective effects in the general population and in patients with chronic kidney disease.[Citation4] Generally, transplant physicians are reluctant to use ACEI’s/ARB’s immediately after transplantation due to the risk of decline in renal function and hyperkalemia and the lack of unequivocal evidence about their long-term effects.[Citation5] On the other hand, many patients received ACEI’s/ARB’s in the treatment period before renal transplantation and it is unclear if the sudden discontinuation of these drug classes has detrimental effects.[Citation6] The aim of this retrospective cohort study was to investigate if the use of ACEI’s/ARB’s (from now on referred to as renin angiotensin aldosterone system blockers-RAASB’s) in the very early postoperative period after renal transplantation is safe and has a positive effect on blood pressure control. Moreover, we wanted to examine if early treatment with RAASB’s has any impact on interstitial fibrosis/tubular atrophy of the graft and investigate long-term effects on patient and graft survival.
Materials and methods
Study population
Patients receiving a kidney transplant from a deceased or living donor at our institution between 2004 and 2011 were retrospectively analyzed. An informed consent was obtained from all patients participating in the protocol biopsy program at the Hannover Medical School. The study population included 142 isolated kidney or combined kidney and pancreas transplant recipients, who had been treated with a RAASB during their initial hospitalization for transplantation. The RAASB was added to the treatment regimen only if blood pressure control could not be achieved with the other antihypertensive agents. 114 kidney transplant patients who did not receive a RAASB served as controls. Patients treated with and without a RAASB were matched via frequency-matching by age, sex, year of transplantation and serum creatinine at the postoperative day where treatment with the RAASB was started. Conductance of the study was approved by the internal ethics review committee. Patient’s records were anonymized and de-identified prior to analysis.
Methods
Patients were eligible, if a RAASB was included in the medication plan at discharge after the index hospitalization, which was the hospitalization where the renal transplantation was performed. The unexposed control group was selected in the same manner (i.e. a RAASB was not included in the medication plan at discharge) and matched to the exposed group treated with a RAASB at discharge according the criteria listed above. Matching by year of transplantation was done to account for temporal trends in the management of renal transplant recipients, like handling blood pressure and immunosuppressive drug target levels. Recorded parameters regarding blood pressure, renal function, potassium levels, weight, use of concomitant antihypertensive or other medications, immunosuppressive drugs and immunosuppressive drug levels over the course of the entire index hospitalization – from postoperative day 1 through the day of discharge – were retrieved for all patients.
Available protocol biopsies conducted 6 and 12 weeks after transplantation were assessed for cortical interstitial fibrosis and tubular atrophy (IFTA). IFTA was semi-quantitatively graded according to the following scoring system: 0–5% = 0, 5–10% = 1, 10–15% = 2, 15–20% = 3, >20% = 4.
Adequacy of blood pressure control (assessed by means of blood pressure measurement at home in the morning and by the sum of antihypertensive agents), renal function, 24 h proteinuria and serum potassium levels at year one after transplantation and at the end of the observational follow-up period were compared between patients treated with and without a RAASB during the index hospitalization.
Lastly, differences in hard clinical end points, such as death from any cause, dialysis and death or dialysis were sought.
For the purpose of the study, all patients of the index hospitalization constituted group I (n = 142 exposed patients, n = 114 unexposed patients). After the analysis of group I, all the above mentioned parameters were compared between those patients of group I who did or did not receive a RAASB continuously for the first year after transplantation. These patients consisted group II (n = 117 exposed patients, n = 50 unexposed patients)
Study objectives
The study was designed to investigate, if the use of RAASB’s in the early postoperative phase after kidney transplantation was efficacious and safe and if this therapeutic maneuver had any effects on relevant post discharge outcomes. For this purpose, renal transplant function, potassium levels and blood pressure control were compared between patients treated with or without a RAASB at discharge from the hospital. Early control biopsies were assessed for cortical fibrosis, assuming that local and systemic beneficial actions of RAASB when introduced very soon after renal transplantation-would be reflected by a lower degree of renal interstitial fibrosis and tubular atrophy.
Furthermore, assuming that many patients treated without RAASB’s at discharge would receive RAASB’s during follow-up and that a RAASB-based therapy at discharge would be interrupted in many other cases, it was interesting to know if the early administration of RAASB’s would have any legacy effects thereafter and if these effects were dependent on the duration of the initial RAASB-based therapy. Accordingly, we first sought if there were any differences in surrogate parameters and hard clinical endpoints in group I considering that in clinical scenario, the patients would differ only by a few weeks of therapy. In a second step, we selected those patients who did or did not receive RAASB’s uninterrupted for the first year after transplantation (group II). Our hypothesis was that a longer duration of initial treatment with RAASB’s would exert more obvious legacy effects.
Statistical analysis
Statistical analyses were performed in SAS 9.3 and SPSS 22. Although multiple tests were performed, the type-I-errors have not been corrected for multiple testing since this is an exploratory epidemiological study. A p value of <0.05 was considered statistically significant.
Exposed (RAASB) and unexposed (no RAASB) patients were compared with respect to their baseline characteristics and several outcome parameters. For continuous variables, the unpaired t-test was used. Differences in skewed continuous variables (as assessed descriptively) were analyzed with Wilcoxon’s rank sum test. For categorical variables, chi-square tests were used to assess differences between the two exposure groups. If not declared otherwise, results in tables are given as mean ± standard deviation and absolute (relative) frequencies, respectively.
In the next step, significant differences in outcome variables had to be adjusted in multivariable analyses using appropriate regression models, i.e. analysis of covariance (ANCOVA) for continuous outcome, logistic regression for binary outcome and Cox proportional hazard regression for time-to-event data. Skewed outcome parameters were log-transformed before ANCOVA. All regression models with RAASB as covariate of primary interest were adjusted for all matching variables (sex, age, year of transplantation, creatinine at day 7). Additionally potential confounders, defined by an association (p < 0.1) with both the exposure (RAASB) and the respective outcome variable, were included to the model. Sensitivity analyses were performed where all variables significantly associated only with the exposure or only with the outcome added to these multivariable regression models.
To illustrate the results, Kaplan–Meier curves were plotted for the time to the occurrence of dialysis/allograft failure and patient death.
Results
Patients of group I and group II
Patients treated with RAASB’s at discharge after the index hospitalization constituted ∼10% of all our renal transplant recipients. Detailed demographic and clinical baseline data of the study population (group I) are listed in . There was a trend to more living donations and significantly less re-transplantation in the RAASB treated patients.
Table 1. Baseline demographic and clinical characteristics in group I and group II.
117 (82.3%) patients were treated with an ACE Inhibitor and 25 (17.6%) with an ARB. Ramipril was used in 111 of 117 and candesartan in 15 of 25 patients. The average starting dose of ramipril was 5mg and of candesartan 16mg. The RAASB was added to the treatment regimen on average at day 8 after transplantation. The mean duration of hospitalization was 13 days and the mean duration of follow-up was 5.6 years (range 0.3–9.2, median 5.8 years) in RAASB’s treated patients and 5.3 years (range 0.4–9.9, median 5.4 years) in patients treated without RAASB’s.
The histological results from the 6 and 12 week protocol biopsies were available for 96 and 89 patients on RAASB’s, respectively, while 72 patients had protocol biopsies at both time points. 83 and 78 patients without RAASB’s had a surveillance biopsy at week 6 and 12 after transplantation. In 70 controls both biopsies had been performed.
117 patients continued to receive the RAASB at year one, in 15 other patients RAASB’s had to be discontinued at a mean time of 11 weeks after transplantation, one patient died and two returned to dialysis. For the remaining seven no data are available regarding the use of RASSB’s at year one. Of the 114 patients without RAASB’s at discharge, 58 were switched to a RAASB at a mean time of 17 weeks after transplantation, whereas only 50 patients continued to be entirely without an RAASB-based therapy during the first year. For the remaining six no data regarding the use of RAASB at year one are available
Baseline clinical data of patients belonging to group II are listed in . Patients using RAASB’s in that group where more likely to receive a kidney from a living donor compared with patients not on RAASB’s. Accordingly, the duration on dialysis and the cold ischemia time was shorter for these patients. The mean duration of follow-up in group II was 5.7 years (range 0.4–9.9, median 5.5 years) in subjects without RAASB and 5.6 years in RAASB treated patients (range 1.6–5.8, median 5.7 years).
Antihypertensive efficacy and safety of RAASB’s during the index hospitalization
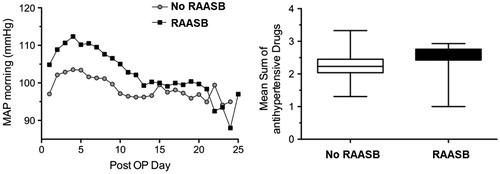
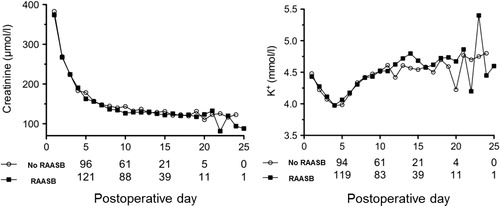
Patients who required a RAASB during the index hospitalization exhibited constantly significantly higher blood pressure values from postoperative day 1 to postoperative day 8 compared with patients without RAASB’s. The difference disappeared quickly after initialization of the RAASB and blood pressure control was then comparable between patients treated with and without a RAASB throughout the entire hospital stay. Nevertheless, RAASB treated patients were in need of more antihypertensive agents (supplementary Table 1 and Figure 1). Estimated GFR, serum creatinine and potassium levels did not differ at any time point over the course of the index hospitalization between patients treated with and without a RAASB ( and ). Importantly, factors that can affect blood pressure, renal function and potassium levels in the early postoperative period, such as dry weight, ciclosporine or tacrolimus levels and use of diuretics did not differ between patients treated with or without a RAASB (data not shown).
Outcomes in group I
Outcome data in group I are presented in supplementary Table 2. There were no statistically significant differences in blood pressure control, but RAASB treated patients required significantly more antihypertensive drugs in the first year. In the RAASB group, creatinine clearance was significantly better at year one, and 24 hour proteinuria was significantly less at 5 years. In the multivariate analysis, the effects on creatinine clearance persisted (p = 0.047 compared to 0.043 in univariate analysis) whereas the effects on proteinuria were lost (p = 0.27).
Table 2. Predictors of dialysis in the univariate analysis (group II).
Generally, interstitial fibrosis and tubular atrophy (IFTA) was negligible in the majority of all patients. The distribution of IFTA was similar in the two groups. There was no difference in any grade of fibrosis between the RAASB and the no RAASB cohort.
There were no significant differences in hard clinical endpoints. Seven (4.9%) patients on RAASB returned to dialysis and seven patients (4.9%) died. Since patients either returned to dialysis or died, a total of n = 14 RAASB patients (9.9%) experienced death or dialysis, whereas the respective numbers of patients without RAASB’s was 7 (6.1%), 10 (8.8%) and 16 (14%) (one patient died after return to dialysis). The differences in RAASB treated and no RAASB treated patients were not statistically significant as assessed by chi-square tests (p = 0.67 for dialysis, p = 0.22 for death and p = 0.30 for death or dialysis). Moreover, time to event analyses for death, dialysis and death or dialysis revealed no significant differences between exposed and unexposed patients. We found a tendency towards protective effects of RAASB intake with hazard ratios of HR = 0.55 for death (p = 0.22), HR = 0.76 for dialysis (p = 0.61) and HR = 0.67 for death/dialysis (p = 0.28).
Outcomes in group II
Outcome data of group II are presented in supplementary Table 3. As in group I, there were no differences in the degree and distribution of IFTA in the 6 and 12 weeks surveillance biopsies. Blood pressure was adequately controlled but RAASB treated patients required significantly more antihypertensive drugs in the first year and at the end of follow-up. The creatinine clearance at year one after transplantation was much better in RAASB treated patients. The difference continued to be significant, albeit at a lower level, after adjustment for baseline parameters (p = 0.01). Renal function, potassium levels and proteinuria at the end of follow-up were similar in patients treated with and without RAASB’s.
Table 3. Predictors of dialysis or death in the univariate analysis (group II).
Five patients (4.3%) on RAASB’s returned to dialysis, five other (4.3%) died and (without overlap of these events) a total of 10 patients (8.5%) experienced death or graft failure, whereas the respective number of patients not being on RAASB’s was seven (14%), four (8%) and 10 (20%). The difference in the endpoints “dialysis” and “death or dialysis” was statistically significant (Odds Ratio = 0.27, p = 0.03 and Odds Ratio = 0.37, p = 0.04). Regarding death, there was no difference in RAASB treated and no RAASB treated patients (p = 0.33). Odds Ratios and p values for dialysis and death/dialysis persisted in the multivariate logistic model adjusted for all matching variables. The additional inclusion of living donation and number of transplantations in the final multivariate model did not alter the results (p = 0.0052 for dialysis and p = 0.0116 for dialysis or death if adjustment for living donation was included and p = 0.0056 for dialysis and p = 0.0133 for dialysis or death if adjustment for living donation and number of transplantations was included).
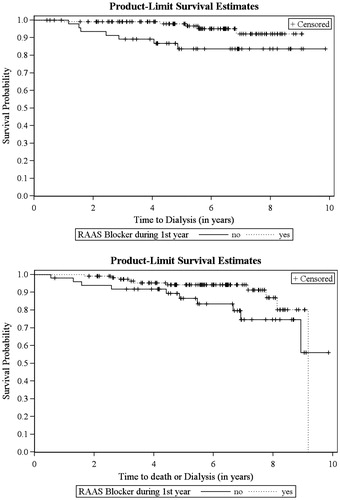
The association of baseline demographic, clinical and immunological risk factors with dialysis and dialysis or death in the univariate cox regression analysis is shown in and the results of the multivariate analysis are presented in . The protective effects of RAASB’s could also be found in the multivariate time-to-event analysis and remained still significant for the time to dialysis (HR = 0.25, p = 0.02, for dialysis; HR = 0.50, p = 0.3 for death and HR = 0.45, p = 0.08 for dialysis or death). The Kaplan–Meier curves for dialysis and dialysis or death are presented in . Most patients were censored in 2013 due to the end of study. The frequency of censoring for reasons of loss-to-follow-up was very low and similar for exposed RAASB patients (4.3%) and unexposed patients (6.0%) in group II as well as in group I.
Table 4. Predictors of dialysis and dialysis or death in the multivariate analysis (group II).
Discussion
The main finding of our study was that RAASB’s are effective antihypertensive agents when used very early after renal transplantation, without jeopardizing renal allograft function and without increasing the risk of hyperkalemia. Furthermore, our study showed that the administration of RAABS’s as early as possible after renal transplantation is renoprotective in the long term and that this happens mainly through blood pressure independent effects.
Physicians are generally reluctant to use RAASB’s prematurely after renal transplantation, for a variety of reasons.[Citation5] The fear of RAASB’s related complications is even greater immediately after renal transplantation, a time period where the resolution of the ischemia reperfusion induced injury of the kidney allograft is eagerly awaited, where the risk of rejection is high and where other potassium sparing medications are administered.[Citation5] Consequently, RAASB’s are usually interrupted preoperatively and re-introduced later at a stage, when the renal transplant function is considered to be more stable. Therefore, it is not surprising that in the most recent randomized controlled studies [Citation7–10] RAASB’s were usually not administered in the first six months after engraftment ().
Table 5. Randomized controlled studies of RAAS blockers after renal transplantation.
One the other hand, blood pressure is often difficult to control in the early postoperative period after renal transplantation, imposing significant risks to the patient and to the graft. Since the range of pressures within which the kidney can autoregulate blood flow is altered in the setting of transplantation, blood pressure extremes can increase the risk of renal injury considerably.[Citation11] Additionally, ischemia reperfusion injury is associated with intrarenal activation of the RAAS [Citation12–14] and withholding of a previous RAASB based therapy leaves the already systemically activated RAAS unopposed acting in the recipient. Thus, uncontrolled hypertension and RAAS activation may be strongly interrelated and potentially detrimental at least for some renal transplant recipients.
The above pathophysiologic considerations, although not systematically tested, are supported by the results of our study. Patients treated with or without RAASB’s did not differ at any factor which could affect blood pressure postoperatively, but the former, exhibited permanently higher blood pressure values, which could only be controlled after the addition of the RAASB in the treatment regimen. Importantly, the decrease in serum creatinine levels after treatment initiation was practically identical between treated and not treated patients and there were no differences in potassium levels at any time point over the course of hospitalization. Our study was the first to record continuously serum creatinine and potassium levels after the initiation of a RAASB-based therapy in a setting of resolving solitary kidney injury and when concomitantly medications which interfere with renal autoregulation, such as the calcineurin inhibitors, are co-administered. Considering this the glomerular capillary pressure would be expected to drop steeper and the consequent increase in serum creatinine could be aggravated.[Citation11] However, no such changes were observed. Similarly, serum potassium remained remarkably unaffected after the introduction the RAASB again under clinical conditions where the risk of hyperkalemia increases significantly. Unfortunately, the interpretation of the results remains only speculative due to the retrospective nature of the study and the absence of physiologic studies. Possible mechanisms in the case of GFR are the inhibition of an overly activated intrarenal RAAS system affecting constriction of the glomerular vasa efferens and vasa afferens as well, the release of intrarenal vasodilatory mediators and renoprotective effects of the better blood pressure control. The absence of hyperkalemia can be attributed to hemodynamic reasons (no GFR decrease) and to increased urinary losses due to the co-existing acute tubular injury. Our findings are further supported by other studies in transplant and non-transplant patients at high renal risk. Acute severe hypertension aggravated kidney injury in patients with preexisting chronic kidney disease,[Citation15] while the withdrawal (compared to continuation) of RAAS blockers before cardiovascular surgery caused more acute renal failure.[Citation16] Similarly, dialysis patients who were treated with RAAS blockers and were subsequently transplanted exhibited early graft dysfunction if the RAAS blocker was discontinued.[Citation6]
Ischemia reperfusion injury elicits an inflammatory response [Citation12,Citation13,Citation17] and therefore, the preliminary use of RAAS blockers could intervene early in specific pathophysiological pathways of the inflammatory cascade and prevent or at least ameliorate acute and chronic allograft injury. The results of our study can be put in the context of the above considerations.
The absence of appreciable differences in interstitial fibrosis and tubular atrophy (IFTA) between cases and controls is in line with other studies with much longer treatment periods.[Citation10] Additionally, 6 weeks surveillance biopsies reflect more donor than recipient related damage,[Citation18] and 12 weeks after transplantation may be a too short time interval, where differences in structural kidney damage can be discernible. Furthermore, the fact that the degree of IFTA was negligible in both groups and at both time points, argues more for good quality organs and for low risk recipients, which makes the demonstration of any differences even more difficult.
The continuous administration of RAASB’s over the entire first year after transplantation prevented graft failure by means of renal-specific, partially blood pressure independent, protective effects. Blood pressure was similar in all patients during follow-up, but patients treated with RAASB’s needed significantly more antihypertensive agents. An assumption – reconciling simultaneously the short- with the long-term results of our study – could be that of a stimulated RAAS, necessitating thereby the prescription of more antihypertensive drugs to control blood pressure.
We could, like others,[Citation10] not demonstrate antiproteinuric actions of RAAS blockers in our cohort. Although proteinuria remains the most important renal prognostic factor and a key treatment target in renal transplant and non-transplant diseases, non-proteinuric renal failure is increasingly considered in the general diabetic population.[Citation19] Besides that, the amount of proteinuria in our study population at the end of follow up was too low in both groups and clinically irrelevant for renal transplant conditions.[Citation5]
Our study was the first to demonstrate that RAASB’s have positive effects on graft survival. Thus far, register analyses have shown neutral [Citation20] or only clear cardiovascular benefits [Citation21] and older small trials demonstrated opposite effects, namely an impairment in GFR after RAASB use.[Citation22] Our results are in line with the well conducted study of Ibrahim et al which demonstrated that RAASB’s did not affect IFTA or IFTA related end stage renal disease but reduced the incidence of all cause end stage renal disease.[Citation10] RAASB’s seem therefore to prolong graft survival. Evidence about possible underlying mechanisms comes again from non-transplant and transplant populations. In ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial), increased baseline plasma renin activity predicted renal impairment but not cardiovascular events,[Citation23] and antibodies against the angiotensin II type 1 receptors have been found to increase the risk of rejection and to affect renal allograft survival.[Citation24,Citation25]
Our study is retrospective with all its inherent drawbacks. Since the main inclusion criterion was the RAASB use only at discharge, patients in whom the RAASB had to be discontinued during hospitalization were not captured and are probably missed. Thus, the results are not generalizable and an early RAASB- based therapy can obviously not be applied to all renal transplant recipients. Nevertheless, the lack of complications in those patients receiving it and the clear positive effects in comparison to a carefully chosen control group, allows us to recommend the early use of RAASB’s in renal transplant recipients with demographic and clinical characteristics similar to the patients of our cohort, when the serum creatinine is decreasing and foremost when uncontrolled hypertension is present.
Because RAAS blocker withdrawal results in rebound effects, one can assume that these rebound effects were responsible for the increase in blood pressure shortly after transplantation. Unfortunately, information about the previous use of RAASB’s or their discontinuation immediately before transplantation is not available for cases and controls. However, we can assume that the rate of patients receiving a RAASB before transplantation was quite similar in both groups, because there was no difference in their cardiovascular or metabolic risk profile. Additionally, our study was a single center study and therefore all dialysis patients had been treated according to the same treatment standards. Admittedly, the renal events were too small to draw definite conclusions or to declare recommendations. But the positive impact of RAASB’s persisted even after adjustment of multiple renal allograft risk factors. In this regard, our results highlight the crucial role of the RAAS system early after renal transplantation. However, they should be viewed only as hypothesis generating and must be ideally confirmed in a prospective randomized controlled trial.
In conclusion, RAASB’s can be administered at a very early time point after renal transplantation in recipients with uncontrolled hypertension and quickly improving renal allograft function. Their use can be considered safe and confers to the graft short and probably long lasting beneficial effects.
Funding information
The work of C. Chatzikyrkou and J. Menne is supported by the European Union (HEALTH-2011-278249-EU-MASCARA).
Supplementary Table
Download MS Word (27.7 KB)Disclosure statement
The authors of this manuscript have no conflicts of interest to disclosure.
References
- Opelz G, Dohler B, Collaborative Transplant Study. Improved long-term outcomes after renal transplantation associated with blood pressure control. Am J Transplant. 2005;5:2725–2731.
- Mange KC, Cizman B, Joffe M, et al. Arterial hypertension and renal allograft survival. JAMA. 2000;283:633–638.
- Collins AJ, Foley RN, Herzog C, et al. US renal data system 2010 annual data report. Am J Kidney Dis. 2011;57:A8, e1–A8, e526.
- Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869.
- Chatzikyrkou C, Menne J, Gwinner W, et al. Pathogenesis and management of hypertension after kidney transplantation. J Hypertens. 2011;29:2283–2294.
- Stevens KK, Patel RK, Clancy M, et al. Angiotensin blockade is associated with early graft dysfunction after live donor renal transplantation. Transplantation. 2010;89:707–709.
- Paoletti E, Cassottana P, Amidone M, et al. ACE inhibitors and persistent left ventricular hypertrophy after renal transplantation: a randomized clinical trial. Am J Kidney Dis. 2007;50:133–142.
- Philipp T, Martinez F, Geiger H, et al. Candesartan improves blood pressure control and reduces proteinuria in renal transplant recipients: results from SECRET. Nephrol Dial Transplant. 2010;25:967–976.
- Paoletti E, Bellino D, Marsano L, et al. Effects of ACE inhibitors on long-term outcome of renal transplant recipients: a randomized controlled trial. Transplantation. 2013;95:889–895.
- Ibrahim HN, Jackson S, Connaire J, et al. Angiotensin II blockade in kidney transplant recipients. J Am Soc Nephrol. 2013;24:320–327.
- Abuelo JG. Normotensive ischemic acute renal failure. N Engl J Med. 2007;357:797–805.
- Correa-Costa M, Azevedo H, Amano MT, et al. Transcriptome analysis of renal ischemia/reperfusion injury and its modulation by ischemic pre-conditioning or hemin treatment. PLoS One. 2012;7:e49569.
- Cavaille-Coll M, Bala S, Velidedeoglu E, et al. Summary of FDA workshop on ischemia reperfusion injury in kidney transplantation. Am J Transplant. 2013;13:1134–1148.
- Sulikowski T, Domanski L, Zietek Z, et al. The effect of preservation solutions UW and EC on the expression of renin I, angiotensinogen and angiotensin I-converting enzyme genes in rat kidney. Ann Transplant. 2011;16:108–113.
- Szczech LA, Granger CB, Dasta JF, et al. Acute kidney injury and cardiovascular outcomes in acute severe hypertension. Circulation. 2010;121:2183–2191.
- Drenger B, Fontes ML, Miao Y, et al. Patterns of use of perioperative angiotensin-converting enzyme inhibitors in coronary artery bypass graft surgery with cardiopulmonary bypass: effects on in-hospital morbidity and mortality. Circulation. 2012;126:261–269.
- Bos EM, Wang R, Snijder PM, et al. Cystathionine γ-lyase protects against renal ischemia/reperfusion by modulating oxidative stress. J Am Soc Nephrol. 2013;24:759–770.
- Mengel M, Chang J, Kayser D, et al. The molecular phenotype of 6-week protocol biopsies from human renal allografts: reflections of prior injury but not future course. Am J Transplant. 2011;11:708–718.
- Macisaac RJ, Jerums G. Diabetic kidney disease with and without albuminuria. Curr Opin Nephrol Hypertens. 2011;20:246–257.
- Opelz G, Zeier M, Laux G, et al. No improvement of patient or graft survival in transplant recipients treated with angiotensin-converting enzyme inhibitors or angiotensin II type 1 receptor blockers: a collaborative transplant study report. J Am Soc Nephrol. 2006;17:3257–3262.
- Heinze G, Mitterbauer C, Regele H, et al. Angiotensin-converting enzyme inhibitor or angiotensin II type 1 receptor antagonist therapy is associated with prolonged patient and graft survival after renal transplantation. J Am Soc Nephrol. 2006;17:889–899.
- Hiremath S, Fergusson D, Doucette S, et al. Renin angiotensin system blockade in kidney transplantation: a systematic review of the evidence. Am J Transplant. 2007;7:2350–2360.
- Sever PS, Chang CL, Prescott MF, et al. Is plasma renin activity a biomarker for the prediction of renal and cardiovascular outcomes in treated hypertensive patients? Observations from the Anglo-Scandinavian cardiac outcomes trial (ASCOT). Eur Heart J. 2012;33:2970–2979.
- Giral M, Foucher Y, Dufay A, et al. Pretransplant sensitization against angiotensin II Type 1 receptor is a risk factor for acute rejection and graft loss. Am J Transplant. 2013;13:2567–2576.
- Taniguchi M, Rebellato LM, Cai J, et al. Higher risk of kidney graft failure in the presence of anti-angiotensin II type-1 receptor antibodies. Am J Transplant. 2013;13:2577–2589.