Abstract
Background: Abnormal P-wave terminal force in lead V1 (PTF-V1) is an ECG marker of increased left atrial (LA) volume, elevated LA filling pressures and/or LA systolic dysfunction. Because left ventricular (LV) diastolic dysfunction is one of the potential mechanisms driving LA remodelling, we hypothesized that PTF-V1 might be an additional ECG marker of diastolic dysfunction.
Methods: LV diastolic function after 3 years’ systematic antihypertensive treatment was examined in relation to baseline PTF-V1 in 431 hypertensive patients undergoing protocol-driven blood pressure reduction who had baseline and year-3 ECG and echocardiographic data and a preserved LV ejection fraction (EF >45%) at year-3. Abnormal diastolic function was defined by the tenth or 90th percentile values from 405 normotensive, non-obese and non-diabetic adults without overt cardiovascular disease. Abnormal PTF-V1, defined by the presence of a negative terminal P-wave in lead V1 ≥ 4000 μV·ms, was present in 167 patients (38.7%).
Results: Abnormal PTF-V1 was associated with worse year-3 mean diastolic first third filling time (0.43 ± 0.08 vs 0.40 ± 0.07 sec, p = 0.039), first half filling time (0.55 ± 0.07 vs 0.53 ± 0.07 sec, p = 0.041), mitral valve A velocity (86 ± 27 vs 76 ± 19 cm/sec, p = 0.009) and mitral valve E/A ratio (0.85 ± 0.22 vs 0.94 ± 0.27, p = 0.007) after adjusting for other potential predictors of diastolic dysfunction including race, and heart rate, systolic blood pressure and severity of ECG LVH by Cornell product criteria at baseline. In parallel multivariate logistic regression analysis, abnormal PTF-V1 was associated with significantly increased odds of abnormal mitral valve E/A ratio (OR 1.55, 95%CI 1.04–2.32 p = 0.032), and a trend toward higher odds of abnormal half filling time (OR 1.42, 95%CI 0.94–2.15, p = 0.098) at year-3 of follow-up.
Conclusions: Abnormal P-wave terminal force in lead V1 is associated with worse diastolic function and predicts abnormal LV diastolic behaviour in patients with preserved EF after 3 years of blood pressure reductive therapy.
Introduction
Hypertension is a significant public health problem affecting ∼30% of men and women in the United States.[Citation1] Left ventricular hypertrophy (LVH) is a well-established cardiac manifestation of hypertension [Citation2–4] and is thought to mediate, at least partially, the relation between hypertension and left atrial (LA) remodelling via left ventricular (LV) diastolic dysfunction.[Citation5,Citation6] As a consequence, LA structural and functional remodelling has been implicated as a potential marker of the duration and severity of diastolic dysfunction.[Citation7,Citation8]
Abnormalities in LV filling and resultant increase in LV end diastolic pressure (LVEDP) are associated with elevated LA pressures and larger LA volumes. Abnormal P-wave terminal force in lead V1 (PTF-V1) is a common observation in hypertensive patients [Citation9] and is a known predictor of increased LA pressure,[Citation10] size,[Citation11] atrial conduction pattern [Citation12,Citation13] and LV mass.[Citation14] Recently, there has been emerging interest surrounding PTF-V1 as a prognostic marker given its association with stroke,[Citation15] major adverse cardiac events [Citation16] and post-myocardial infarction (MI) death or heart failure (HF).[Citation17] Diastolic dysfunction provides a potential pathophysiologic mechanism linking abnormal PTF-V1 to many of these epidemiological outcomes, including diastolic HF.
Other than electrocardiographic criteria for LVH, there are no other known electrocardiographic (ECG) predictors of diastolic dysfunction.[Citation18] As a product of the growing burden of hypertension and diabetes in the population, there has been a significant increase in the prevalence of HF with preserved ejection fraction (HFpEF). Therefore, a cost-effective means to identify patients at risk of developing HFpEF is of importance. We hypothesized that PTF-V1 might be an additional ECG marker of diastolic dysfunction given its association with LA manifestations of abnormal diastolic function. To test this hypothesis, we examined echocardiographic LV diastolic function in hypertensive patients enrolled in the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) study in relation to the presence or absence of abnormal PTF-V1.
Methods
Study population
The LIFE trial was a prospective randomized double-blind study that enrolled 9193 hypertensive patients with ECG LVH by Cornell voltage-duration product and/or Sokolow-Lyon voltage criteria.[Citation19,Citation20] As described in detail elsewhere,[Citation19,Citation20] eligible patients for LIFE were 55–80 years of age with essential hypertension with blood pressures ranging from 160 to 200/95 to 115 mmHg off all antihypertensive therapy for one to two weeks who did not have a myocardial infarction or stroke within 6 months of enrollment, severe LV dysfunction, HF, or angina requiring additional treatment with an antihypertensive agent (i.e. ACE inhibitor, AT1 antagonist or beta-blocker). A total of 964 patient sub-group of the LIFE study underwent baseline echocardiograms, of whom 431 had baseline and year-3 ECG and echocardiographic data, and a preserved LV ejection fraction (>45%). These 431 participants (247 male, 184 female) are included in this study.
Treatment regimens
Blinded placebo-controlled treatment was initiated with losartan 50 mg or atenolol 50 mg daily with a target BP <140/90 mm Hg. Therapy could be titrated during the study period with the addition of hydrochlorothiazide 12.5mg, followed by blinded upward titration of atenolol or losartan to 100 mg daily if needed. Additional open-label upward titration of hydrochlorothiazide, and if necessary initiation of therapy with a calcium channel blocker or other antihypertensive therapies (excluding AT blockers, beta-blockers, or ACE inhibitors), was allowed if indicated to control the high BP.[Citation20]
Echocardiography
Echocardiographic procedures and measurements were performed as previously described in detail.[Citation21–24] LV dimensions, wall thickness and diastolic parameters were measured at end-diastole according to the American Society of Echocardiography recommendation.[Citation25] Diastolic function variables included: mitral valve E velocity (MV E), A velocity (MV A), E/A ratio (MV E/A), acceleration time (MV atime), deceleration time (MV decel time), diastolic filling time (MV filling time), half filling time (MV half-time), third filling time (MV third-time), atrial filling fraction (MV atrial filling fraction) and isovolumic relaxation time (IVRT). Measurements for up to three cardiac cycles were averaged. Tenth or 90th percentile values from 405 normotensive, non-obese, non-diabetic adults without overt cardiovascular disease were used to define abnormal diastolic function [Citation21] with the exception of MV E/A for which the year-3 median value was used as a cut-point to define abnormal.
Electrocardiography
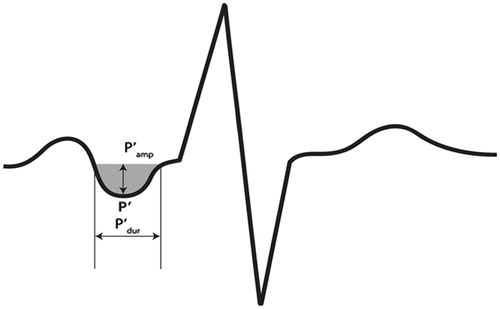
Electrocardiograms were obtained at baseline, 6-months, and at yearly intervals until study termination or patient death as previously reported.[Citation26,Citation27] Abnormal PTF-V1, defined by the presence of a negative terminal P-wave in lead V1 with amplitude × duration ≥4000 μV·ms (), was determined on baseline ECGs as part of a LIFE sub-study of QT interval behaviour.
Statistical analysis
All statistical analyses were performed using SPSS version 22.0 (SPSS Inc., Chicago, IL). Continuous variables are presented as mean ± SD and categorical variables are expressed as percentages. Patients were grouped according to the presence or absence of abnormal PTF-V1 at baseline. Differences between groups were analyzed using unpaired samples Student’s t-tests and analysis of covariance for continuous variables and χ2 analysis for discrete variables. Univariate logistic regression analyses using abnormal diastolic variables as dependent variables were used to further define the relationship between abnormal PTF-V1 and diastolic dysfunction. Multivariate logistic regression was used to adjust for other univariate predictors of diastolic dysfunction including heart rate and systolic blood pressure at baseline, and race. Given that prior work identified severity of ECG LVH by Cornell product criteria as a univariate predictor of diastolic function,[Citation18,Citation28] adjustment for Cornell product at baseline was also included in the multivariate logistic regression analysis. Two-tailed p values less than 0.05 were considered significant for all tests.
Results
At baseline, there was abnormal PTF-V1 identified in 167 patients (39.8%). illustrates baseline demographic and clinical characteristics in relation to the presence or absence of abnormal PTF-V1. Compared to patients with normal PTF-V1, patients with abnormal PTF-V1 were more likely to be black, but were similar with respect to age, gender, randomized treatment allocation, baseline body mass index, total cholesterol levels, HDL cholesterol, serum glucose and urine albumin/creatinine ratio.
Table 1. Baseline demographic and clinical characteristics in relation to the presence of abnormal P-terminal force at baseline.
Measurements of blood pressure, heart rate and ECG LVH at baseline and change from baseline to year-3 in relation to the presence or absence of abnormal PTF-V1 at baseline are shown in . Patients with abnormal PTF-V1 had significantly higher systolic blood pressure and heart rate at baseline, with trends towards lower baseline Sokolow-Lyon voltage and smaller reduction in Sokolow-Lyon voltage between baseline and year 3.
Table 2. Baseline and change from baseline to year-3 blood pressure, heart rate and electrocardiographic left ventricular hypertrophy in relation to the presence or absence of abnormal P-terminal force at baseline.
Mean year-3 echocardiographic LV diastolic function in relation to the presence or absence of abnormal PTF-V1 on baseline ECG is examined in . In unadjusted analyses, patients with abnormal PTF-V1 had significantly worse diastolic function as measured by mean MV filling time, MV half-time, MV third-time, MV atrial filling fraction, MV A and MV E/A, but were similar with respect to other filling parameters. Because patients with and without abnormal PTF-V1 at baseline differed with respect to a number of demographic and clinical factors that could influence LV diastolic function over time, the relation of mean values of LV diastolic function at year-3 with unadjusted significant p values (p < 0.05) to the presence or absence of abnormal PTF-V1 at baseline were examined after adjusting for differences in race, and heart rate, systolic blood pressure and severity of ECG LVH by Cornell product criteria at baseline using analysis of covariance (). After this adjustment, mean diastolic function remained significantly worse as measured by MV half-time, MV third time, MV A velocity and MV E over A velocity. However, univariate differences in mean values of MV filling time and MV atrial filling fraction were attenuated after multivariable adjustment, with a trend towards worse diastolic function by MV atrial filling fraction (p = 0.089).
Table 3. Mean year-3 left ventricular diastolic variables to the presence or absence of abnormal P-terminal force at baseline.
The prevalence of year-3 abnormal values of diastolic function in relation to the presence or absence of abnormal PTF-V1 at baseline is illustrated in . Patients with abnormal PTF-V1 had a significantly higher prevalence of abnormal diastolic function as measured by all variables except MV E, MV acceleration time, MV deceleration time, MV diastolic filling time and IVRT. There was however, a trend toward worse MV diastolic filling time in patients with abnormal PTF-V1.
Table 4. Prevalence of year-3 abnormal left ventricular diastolic variables to abnormal P-terminal force at baseline.
examines the relationship of abnormal diastolic function at year 3 to abnormal PTF-V1 at baseline for year-3 LV diastolic variables in which mean differences were statistically significant after analysis of covariance (). In univariate logistic regression analyses, patients with abnormal PTF-V1 had between 1.63- and 1.82-fold higher odds of abnormal diastolic function as measured by MV half-time, MV third-time and MV A and MV E/A ratio. In multivariate logistic regression analyses adjusting for univariate predictors of diastolic dysfunction including race, and heart rate and systolic blood pressure at baseline, as well as for baseline severity of ECG LVH by Cornell product criteria, abnormal baseline PTF-V1 remained associated with significantly increased odds of abnormal mitral valve E/A ratio and a trend toward higher odds of abnormal half-filling time with attenuation of the relationship with the other diastolic parameters ( and ).
Figure 2. MVhalft: mitral valve half filling time; MVthirdt: mitral valve third filling time; MVaff: mitral valve atrial filling fraction; MVa: mitral valve A velocity; MVea: mitral valve E/A ratio. *Adjusted for race, and heart rate, systolic blood pressure and Cornell product at baseline.
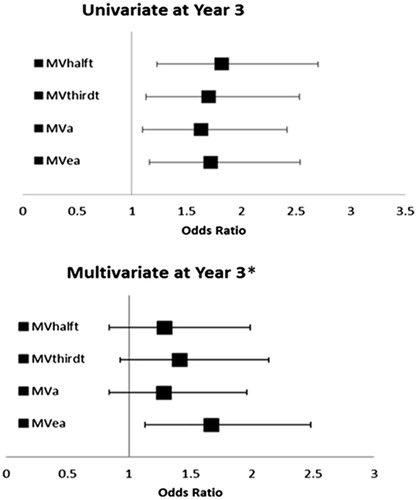
Table 5. Univariate and multivariate logistic regression analyses for risk of having abnormal diastolic function at year-3 in relation to the presence or absence of abnormal P-terminal force at baseline.
Discussion
Our findings demonstrate that in hypertensive patients undergoing blood pressure reducing therapy, abnormal PTF-V1 is associated with significantly worse mean LV diastolic function assessed by a number of measures and modestly predicts abnormal diastolic function in hypertensive patients with preserved EF. Independent of other potential predictors of diastolic dysfunction, abnormal baseline PTF-V1 remained associated with significantly increased risk of abnormal diastolic function as measured by MV E/A ratio, with a trend toward higher odds of abnormal MV half filling time at year-3. These findings suggest that abnormal diastolic function is a plausible pathophysiologic mechanism that links abnormal PTF-V1 to increased risk of diastolic HF.
Previous work has established the association between hypertension and increased LV mass.[Citation29,Citation30] The Framingham Heart Study group was the first to demonstrate a relation between the hypertension and LA size, and further speculated that LVH is a partial mediator of hypertension and LA enlargement.[Citation5] Later work further built upon this hypothesis, demonstrating associations between increased LV mass, diastolic dysfunction and atrial dimension in hypertensive patients.[Citation23,Citation29,Citation30]
Historically, the presence of abnormal PTF-V1 was thought to be a marker of “atrial involvement” in patients with LVH.[Citation31] It is now known that PTF- V1 predicts LA size,[Citation11,Citation32] intra-atrial pressure [Citation10] and atrial conduction pattern.[Citation13] Recent work has demonstrated an association between abnormal PTF-V1 and the presence of LV fibrosis on cardiac magnetic resonance (CMR),[Citation12] and has established PTF-V1 as a prognostic marker of sudden cardiac death (SCD),[Citation33] MACE outcomes in patients presenting with non-ST-segment elevation acute coronary syndrome, and hospitalizations for HF.[Citation16] While the aforementioned studies included patients with abnormal PTF-V1 at increased risk for diastolic dysfunction, none evaluated the association directly.
The present study extends previous findings by demonstrating a modest association between depressed parameters of diastolic function on echocardiogram and abnormal PTF-V1 in patients undergoing blood pressure lowering therapy. Additionally, our work demonstrates significantly worse mean measures of LV diastolic function in patients with abnormal PTF-V1 independent of possible confounding effects of differences in race, and heart rate, systolic blood pressure and severity of ECG LVH by Cornell product criteria at baseline. However, our results also indicate that the increased odds of abnormal diastolic function in patients with abnormal PTF-V1 is contingent on contributing cofactors given the attenuation of the relationship with all diastolic parameters except MV E/A ratio. These findings confirm what is known about correlates of LA size in hypertensive patients with LVH,[Citation6] and add race and heart rate to the list of factors that may mediate the relationship between PTF-V1 and diastolic dysfunction. Furthermore, in contrast to prior work in LIFE that focuses on the relationship of LA size and diastolic function by echo, this study is the first to evaluate the relationship between abnormal PTF-V1 on 12-lead ECG and echocardiographic measures of diastolic function.
There are several potential mechanisms linking abnormal PTF-V1 to depressed diastolic function. A recent study of 91 patients with underlying heart disease in the absence of atrial fibrillation (AF) undergoing CMR demonstrated a significant association between increasing deciles of LV fibrosis (95%CI −1.42 to −0.09; p = 0.025) as well as higher LA volumes and lower LA emptying fractions in patients with abnormal PTF-V1.[Citation12] Additional work by Liu et. al [Citation17] found a significant association between PTF-V1 and cardiac death or hospitalization for heart failure at 6-year follow-up in patients with prior MI (HR 2.72, 95%CI 1.24–5.99, p = 0.01). A later study by Tereschencko et al. illustrated the feasibility of PTF-V1 as a prognostic marker for risk of sudden cardiac death (SCD) (HR 2.49, 95%CI 1.51–4.10).[Citation33] Thus, a decrease in LV diastolic function as a result of LV fibrosis, LVM and/or LVH may be related, at least in part, to the development of abnormal PTF in lead V1. The physiologic mechanism by which this occurs is likely mediated by increased LA volumes, decreased LA emptying and unfavourable LA remodelling as a consequence of atrial contraction against a stiffened LV. Taken together with the known predictive value of diastolic dysfunction for HF,[Citation34,Citation35] these findings suggest a potential predictive value of PTF-V1 in HF risk assessment.
The current study has several potential limitations that merit attention. First, the LIFE study and its echocardiographic subset is a selected population based on the presence of moderate–severe hypertension and of ECG LVH by Cornell product and Sokolow-Lyon voltage criteria. Therefore, the baseline risk of the study sample is increased relative to patients with less severe hypertensive disease possibly making the results less representative of other populations. However, the number of adults meeting entry criteria for the LIFE study has been estimated at 7.8 million in the first 15 member states of the European Union [Citation36] with nearly as many in either the United States or Eastern Europe. Second, PTF-V1 measurements were not documented at year 3. As such, it is unclear how P-wave morphology might change as a consequence of improvement in LV structure and function during antihypertensive treatment. Third, given that age strongly predicts diastolic dysfunction [Citation18,Citation34] and that antihypertensive treatment results in improvement of diastolic filling parameters,[Citation4] the use of baseline PTF-V1 in relation to year-3 diastolic measures on echocardiogram may have underrepresented the association. Finally, our results need be interpreted in the face of known limitations of 12-lead electrocardiogram. Despite these limitations, we were able to demonstrate a clear relationship between abnormal PTF-V1 and diastolic dysfunction on echocardiogram.
This study has several potentially important implications. Our study validates an underlying mechanism by which abnormal P-wave index occurs and further delineates a pathophysiologic mechanism that reflects interplay between LV anatomy and diastolic function, and its influence on LA electrophysiology. Additionally, given the rising cost of healthcare and its significant impact on global government expense, identification of a cost-effective method of identifying at-risk patients is of high importance. Our findings suggest that in addition to determining risk of stroke, cardiac death and HF, ECG assessment of PTF-V1 in hypertensive patients may provide useful data regarding the presence of diastolic abnormalities that could lead to prognostic information pertaining to HF risk. Additional study is needed to further test our hypothesis and elucidate the association between abnormal PTF-V1 and diastolic HF risk. Furthermore, given the known association between LVM reduction and improvement of diastolic filling parameters during antihypertensive therapy,[Citation4] additional work to determine whether serial assessment of PTF-V1 is associated with changing severity of abnormal diastolic function might be of interest. Finally, advanced imaging modalities such as CMR may prove to be a useful method to further delineate the relative contributions of increased LVM, LVH and myocardial fibrosis to diastolic performance over time, and its relation to abnormal terminal P-wave force on electrocardiogram.
Acknowledgments
Drs. Tanoue, Kjeldsen, Devereux, and Okin, had full access to all of the data in the study and take responsibility for the integrity accuracy of the data analysis. Study concept and design: Tanoue, Kjeldsen, Devereux, and Okin. Acquisition of data: LIFE study group. Statistical analysis: Tanoue and Okin. Analysis and interpretation of data: Tanoue and Okin. Manuscript drafting: Tanoue and Okin. Critical revision of the manuscript: Kjeldsen, Devereux, and Okin. Federal and industry support: none.
Disclosure statement
The authors have the following disclosures to report: Dr. Okin has served as a consultant for Novartis and Dr. Kjeldsen has received honoraria from Bayer, Merck, Sharp & Dohme and Takeda, and served as a consultant for Bayer and Takeda.
References
- Wang Y, Wang QJ. The prevalence of prehypertension and hypertension among US adults according to the new joint national committee guidelines: new challenges of the old problem. Arch Intern Med. 2004;164:2126–34.
- Kannel WB, Castelli WP, McNamara PM, et al. Role of blood pressure in the development of congestive heart failure. The Framingham study. N Engl J Med. 1972;287:781–7.
- Koren MJ, Devereux RB, Casale PN, et al. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114:345–52.
- Wachtell K, Bella JN, Rokkedal J, et al. Change in diastolic left ventricular filling after one year of antihypertensive treatment: The Losartan Intervention For Endpoint Reduction in Hypertension (LIFE) study. Circulation. 2002;105:1071–6.
- Vaziri SM, Larson MG, Lauer MS, et al. Influence of blood pressure on left atrial size. The Framingham Heart Study. Hypertension. 1995;25:1155–60.
- Gerdts E, Oikarinen L, Palmieri V, et al. Losartan Intervention For Endpoint Reduction in Hypertension S. Correlates of left atrial size in hypertensive patients with left ventricular hypertrophy: the Losartan Intervention For Endpoint Reduction in Hypertension (LIFE) Study. Hypertension. 2002;39:739–43.
- Tsang TS, Barnes ME, Gersh BJ, et al. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol. 2002;90:1284–9.
- Teo SG, Yang H, Chai P, et al. Impact of left ventricular diastolic dysfunction on left atrial volume and function: a volumetric analysis. Eur J Echocardiogr. 2010;11:38–43.
- Tarazi RC, Miller A, Frohlich ED, et al. Electrocardiographic changes reflecting left atrial abnormality in hypertension. Circulation. 1966;34:818–22.
- Chandraratna PA, Hodges M. Electrocardiographic evidence of left atrial hypertension in acute myocardial infarction. Circulation. 1973;47:493–8.
- Miller DH, Eisenberg RR, Kligfield PD, et al. Electrocardiographic recognition of left atrial enlargement. J Electrocardiol. 1983;16:15–22.
- Tsao CW, Josephson ME, Hauser TH, et al. Accuracy of electrocardiographic criteria for atrial enlargement: validation with cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2008;10:7.
- Josephson ME, Kastor JA, Morganroth J. Electrocardiographic left atrial enlargement. Electrophysiologic, echocardiographic and hemodynamic correlates. Am J Cardiol. 1977;39:967–71.
- Morris JJ, Jr., Estes EH, Jr., Whalen RE, et al. P-wave analysis in valvular heart disease. Circulation. 1964;29:242–52.
- Kamel H, Soliman EZ, Heckbert SR, et al. P-wave morphology and the risk of incident ischemic stroke in the multi-ethnic study of atherosclerosis. Stroke. 2014;45:2786–8.
- Li Q, Gu LD, Zhang C, et al. A predictive study of the dynamic development of the P-wave terminal force in lead V1 in the electrocardiogram in relation to long-term prognosis in non-ST-segment elevation acute coronary syndrome patients during hospitalization. Ann Noninvasive Electrocardiol. 2015;20:542–53.
- Liu G, Tamura A, Torigoe K, et al. Abnormal P-wave terminal force in lead V1 is associated with cardiac death or hospitalization for heart failure in prior myocardial infarction. Heart Vessels. 2013;28:690–5.
- Krepp JM, Lin F, Min JK, et al. Relationship of electrocardiographic left ventricular hypertrophy to the presence of diastolic dysfunction. Ann Noninvasive Electrocardiol. 2014;19:552–60.
- Okin PM, Devereux RB, Harris KE, et al. Regression of electrocardiographic left ventricular hypertrophy is associated with less hospitalization for heart failure in hypertensive patients. Ann Intern Med. 2007;147:311–9.
- Dahlöf B, Devereux RB Kjeldsen SE. Group LS, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003.
- Bella JN, Palmieri V, Liu JE, et al. Relationship between left ventricular diastolic relaxation and systolic function in hypertension the Hypertension Genetic Epidemiology Network (HyperGEN) Study. Hypertension. 2001;38:424–8.
- Wachtell K, Bella JN, Liebson PR, et al. Impact of different partition values on prevalences of left ventricular hypertrophy and concentric geometry in a large hypertensive population: the LIFE study. Hypertension. 2000;35:6–12.
- Wachtell K, Smith G, Gerdts E, et al. Left ventricular filling patterns in patients with systemic hypertension and left ventricular hypertrophy (the LIFE study). Losartan Intervention For Endpoint. Am J Cardiol. 2000;85:466–72.
- Devereux RB, Bella J, Boman K, et al. Echocardiographic left ventricular geometry in hypertensive patients with electrocardiographic left ventricular hypertrophy: the LIFE study. Blood Press. 2001;10:74–82.
- Lang RM, Bierig M, Devereux RB, et al. Chamber quantification writing G, American society of echocardiography's G, standards C, European association of E. Recommendations for chamber quantification: a report from the American society of echocardiography's guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European association of echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63.
- Muiesan ML, Salvetti M, Monteduro C, et al. Changes in midwall systolic performance and cardiac hypertrophy reduction in hypertensive patients. J Hypertens. 2000;18:1651–6.
- Gerdts E, Okin PM, Boman K, et al. Association of heart failure hospitalizations with combined electrocardiography and echocardiography criteria for left ventricular hypertrophy. Am J Hypertens. 2012;25:678–83.
- Tanoue MT, Kjeldsen SE, Devereux RB, et al. Relationship of diastolic function to new or persistent electrocardiographic left ventricular hypertrophy. Blood Press. 2016;1–9. [Epub ahead of print].
- Gerdts E, Lund-Johansen P, Omvik P. Factors influencing left ventricular mass in salt sensitive and salt resistant essential hypertensive patients. Blood Press. 1998;7:223–30.
- Hammond IW, Devereux RB, Alderman MH, et al. Relation of blood pressure and body build to left ventricular mass in normotensive and hypertensive employed adults. J Am Coll Cardiol. 1988;12:996–1004.
- Romhilt DW, Bove KE, Conradi S, et al. Morphologic significance of left atrial involvement. Am Heart J. 1972;83:322–7.
- Jin L, Weisse AB, Hernandez F, et al. Significance of electrocardiographic isolated abnormal terminal P-wave force (left atrial abnormality). An echocardiographic and clinical correlation. Arch Intern Med. 1988;148:1545–9.
- Tereshchenko LG, Henrikson CA, Sotoodehnia N, et al. Electrocardiographic deep terminal negativity of the P wave in V1 and risk of sudden cardiac death: the Atherosclerosis Risk in Communities (ARIC) study. J Am Heart Assoc. 2014;3:e001387.
- Redfield MM, Jacobsen SJ, Burnett Jr., JC, et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202.
- Vasan RS, Benjamin EJ, Levy D. Prevalence, clinical features and prognosis of diastolic heart failure: an epidemiologic perspective. J Am Coll Cardiol. 1995;26:1565–74.
- Dahlöf B, Burke T, Krobot K, et al. Population impact of losartan use on stroke in the European Union (EU): projections from the Losartan Intervention For Endpoint reduction in hypertension (LIFE) study. J Hum Hypertens. 2004;18:367–73.