Abstract
The main Hypertension in the Very Elderly Trial (HYVET) demonstrated a very marked reduction in cardiovascular events by treating hypertensive participants 80 years or older with a low dose, sustained release prescription of indapamide (indapamide SR, 1.5 mg) to which was added a low dose of an angiotensin converting enzyme inhibitor in two-thirds of cases (perindopril 2–4 mg). This report from the ambulatory blood pressure sub-study investigates whether changes in arterial stiffness and ambulatory blood pressure (BP) could both explain the benefits observed in the main trial. A total of 139 participants were randomized to placebo [67] and to active treatment [72] and had both day and night observations of BP and arterial stiffness as determined from the Q wave Korotkoff diastolic (QKD) interval. The QKD interval was 5.6 ms longer (p = 0.017) in the actively treated group at night than in the placebo group. This was not true for the more numerous daytime readings so that 24-h results were similar in the two groups. The QKD interval remained longer at night in the actively treated group even when adjusted for systolic pressure, heart rate and height. The reduced arterial stiffness at night may partly explain the marked benefits observed in the main trial.
Introduction
Arterial stiffness is associated with an increase in blood pressure (BP). Greater stiffness has also been shown to be related to an increase in cardiovascular events, independent of BP.[Citation1,Citation2] There is evidence that thiazide diuretics have little effect on arterial stiffness [Citation3] but little is known of the effects of non-thiazide diuretics such as indapamide and chlorthalidone. Angiotensin converting enzyme inhibitors (ACE-I) reduce arterial stiffness and, in one randomized trial, the combination of perindopril (an ACE-I) and indapamide reduced augmentation index (AI x) compared to atenolol but not arterial pulse wave velocity (A-PWV).[Citation4]
The lack of effect on A-PWV may have been due to atenolol also reducing A-PWV.[Citation4–6] In the Hypertension in the Very Elderly Trial (HYVET), the effect on 24-h BP and arterial stiffness with indapamide and perindopril was compared with matched placebo in very elderly (more than or equal to 80 years) hypertensive subjects.[Citation7] A measure of arterial stiffness, the Q wave Korotkoff diastolic (QKD) interval (time interval between the QRS wave on the electrocardiogram (ECG) and the detection of the last Korotkoff sound during BP measurement over the brachial artery) is closely related to A-PWV [Citation8] and was explored in a 24-h ambulatory blood pressure sub-study.[Citation9] Since QKD measures the time taken for the pulse wave to travel from the heart to the recording point, it is negatively correlated with the velocity as given by PWV and is also negatively correlated with arterial stiffness. This means that a higher QKD is associated with a lower arterial stiffness and vice versa. The results of the sub-study provided information on the effects of indapamide ± perindopril on arterial stiffness and its circadian variation.
Method
The protocol for ambulatory blood pressure (ABP) and QKD monitoring in HYVET has been published elsewhere,[Citation9] as has the protocol for the main study (registered trial number NCT00122811) and the main results.[Citation10] Participants were eligible for the trial if they were aged ≥80 years and had an average systolic clinic blood pressure (CBP) of 160–199 mm Hg after at least a two-month placebo single blind run-in and an average standing systolic CBP of ≥140 mm Hg. At the start of the trial, the participants had to have a diastolic CBP of 90–109 mm Hg, although this criterion was later relaxed to allow the inclusion of subjects with isolated systolic hypertension and diastolic clinic CBP <90 mm Hg. Treatment was started with indapamide sustained release (SR) 1.5 mg daily or matching placebo, to which could be added perindopril 2 or 4 mg daily (or matching placebo) to achieve a goal of systolic CBP <150 mm Hg and diastolic CBP <80 mm Hg.
ABP readings were recorded by the validated Diasys Integra II ABP recording machine.[Citation11,Citation12] This machine recorded auscultatory readings if possible and oscillometric readings when the auscultatory readings were not satisfactory. Auscultatory readings were recorded in 86% of instances. The Diasys Integra II also measures arterial stiffness using the QKD interval [Citation13] and relies on auscultatory readings for this measure. The reproducibility of QKD measurements has been reported to be satisfactory.[Citation14] This machine was selected as it provides a 24-h measure that includes the cardiac pre-ejection period and, most importantly, a transit time related inversely to pulse wave velocity.
It was planned to recruit 600 patients into the sub-study with data both at baseline and during the double-blind phase of the trial. Unfortunately, this was not achieved, though 284 were recruited. Of these, 36 were excluded for the following reasons: 11 recorded <60% of possible auscultatory readings, 3 were studied while no longer on trial medication, and 1 was excluded because of uncontrolled atrial tachycardia. For baseline measurements, 7 were excluded because they were studied within 7 days of starting placebo run-in and were previously on antihypertensive treatment; and 14 were studied after randomization and may have started trial medication. This left 248 patients in the sub-study (112 studied at baseline and 186 on double-blind treatment after about one year). The communication considers the 186 studied on treatment (or placebo). Of these, 139 had both day and night-time readings recorded and allowed the investigation of circadian rhythm (67 on placebo and 72 on active treatment). The required number of readings in each period and other quality control issues were previously described.[Citation9] Baseline measurements of QKD were only available in 50 participants who had measurements during treatment and therefore, adjustment was not made for baseline values.
Daytime ambulatory readings were those recorded between 8:00 am and 8:00 pm. (The time 8:00 pm was chosen because some elderly participants retire to bed early.) Night-time readings were recorded between 10:00 pm and 6:00 am. (The time 6:00 am was chosen in view of the tendency in the very elderly to sleep a shorter number of hours.)
Statistical methods
The QKD data were analysed using linear mixed models. These models are useful where repeated measurements are made on the same subject, as in this study, and have the advantage of dealing with missing values. Subject was treated as a random effect, and treatment (active or placebo), and time of day (day or night) were fixed effects. An interaction term treatment multiplied by day-night difference was included to determine if treatment influenced the circadian rhythm of QKD. Systolic BP, heart rate and height are known to be associated with QKD and therefore, they were also included in the model. The analysis was carried out using SAS/STAT software (SAS Institute Inc., Cary, NC). p Values less than 0.05 were deemed to indicate statistical significance.
Results
Of the 67 participants receiving a placebo, 69% were female and their average age was 84 ± 3 years. Similarly, of the 72 receiving active treatment, 64% were female and their average age was 84 ± 6 years. Height ranged from 145 to 183 cm (mean 161 cm) in those given placebo and 138 to 185 cm (mean 160 cm) in those randomized to active treatment.
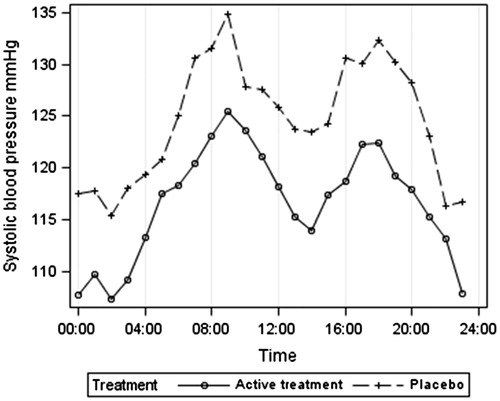
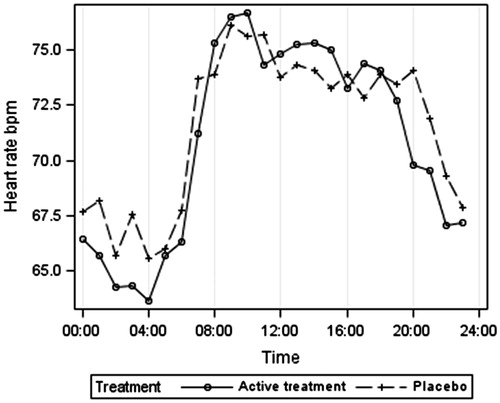
gives the results after an average of 13-month placebo or active treatment. Both ambulatory systolic and diastolic BP were lower at night than during the day (by an average of 10 mm Hg systolic and 6 mm Hg diastolic pressure). Systolic BP was lower on active treatment than placebo by an average of 8 mm Hg systolic and 6 mm Hg diastolic. Heart rate was lower by night than during the day by an average of nine beats per minute. QKD was similar in placebo and active treated groups during the day but QKD was higher at night on active treatment compared with placebo by 5.6 ms.
Table 1. Means (standard deviations) after an average of 13-month placebo or active treatment.
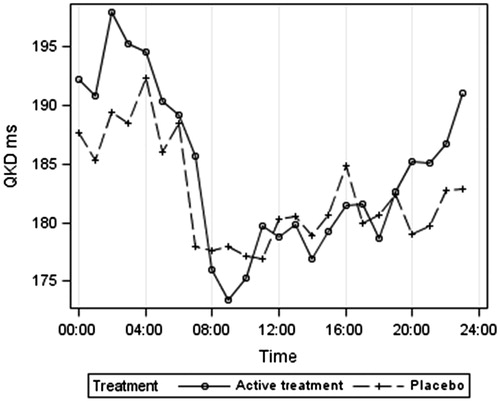
gives the ambulatory systolic BP according to treatment group. The lower BP at night is clearly shown for both treatment groups, as is the postprandial dip, which was marked in these very elderly hypertensive patients, averaging 11 mm Hg between the morning peak at 09:00 and 14:00 h. gives the 24-h heart rate results, indicating a higher heart rate in the placebo group at night. illustrates the 24-h QKD results and shows the higher average QKD results at night in the active treatment group. confirms these results after adjustments for systolic BP, heart rate and height. The mixed model results () showed the importance of adjusting for systolic BP (p < 0.001), height (p = 0.006) and possibly heart rate (p = 0.089). QKD also varied between day and night (p = 0.002) being longer at night. Although no significant overall effect of treatment was found, there was a significant interaction between day–night and treatment (p = 0.017), independent of other variables, suggesting that the difference between treatments depends on whether it is day or night and supporting the finding of a longer QKD (lower stiffness) at night in the actively treated group.
Figure 4. Q wave Korotkoff diastolic interval (QKD) adjusted for systolic blood pressure, heart rate, and height, according to treatment group.
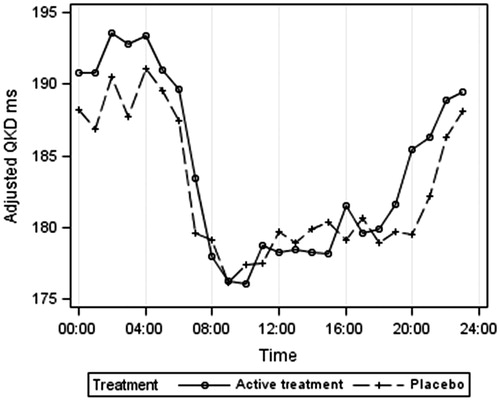
Table 2. Summary results from the normal mixed model analysis, outcome variable = QKD (ms).
Discussion
Over the 24-h period, QKD and therefore arterial stiffness were similar in the actively and placebo treated groups. However, there was a marked circadian rhythm with higher stiffness (lower QKD) during the day. The QKD (uncorrected for systolic BP and heart rate) was longer in the actively treated group at night (). A higher QKD may be driven by a lower systolic BP and heart rate. However, correction for these factors () did not remove this effect and the statistical model demonstrated a difference in circadian rhythm between the two treatment groups (p = 0.017) with higher adjusted QKD at night in the actively treated group balanced by a slightly lower adjusted QKD during the day.
Which is the better predictor of cardiovascular events, a higher arterial stiffness during the daytime or at night? With respect to BP, a recent meta-analysis concluded that a higher night-time BP is a better predictor for adverse outcome than a higher day-time BP or even a low day–night BP ratio.[Citation15] Specifically a high night-time BP rather than day-time BP is associated with chronic kidney disease,[Citation16] left ventricular hypertrophy [Citation17] and cardiovascular disease.[Citation18–20]
Cuspidi and Grassi [Citation21] conclude that nocturnal BP should be targeted by treatment.
In the actively treated group, systolic BP was 8/5 mm Hg lower at night and pulse rate 1.4 beats per minute lower than in the placebo group. However, this could not explain the higher QKD at night of 5.6 ms in the actively treated group. QKD was not closely related to BP and although and show a good inverse relation between QKD and heart rate, and do not show such a clear relation between QKD and BP, the dip in BP between 10:00 and 15:00 h not being associated with any change in QKD. Moreover, during the day BP was 8/7 mm Hg lower in the actively treated group but QKD tended to average 1.5 ms lower. Finally adjustments for systolic BP did not negate the prolonged QKD at night in the active group (). We conclude that indapamide + perindopril favourably affected arterial compliance at night independently from BP.
As reducing BP at night is especially beneficial it is possible that lowering arterial compliance at night produces extra benefits. This possibility is underlined by the very positive results of the main HYVET trial.[Citation10] As two-thirds of the participants in the HYVET trial had both indapamide and perindopril, it is not possible to ascribe the potential benefits to one drug or the other. The combination has been shown to reduce A-PWV (4) although one study claimed that neither a thiazide nor an ACE inhibitor reduces PWV (5). A further study claimed that an ACE inhibitor could reduce brachial-radial PWV by 17% [Citation22] and the possibility that ACE inhibitors increase large artery compliance is supported by many authors.[Citation23–28]
PWV is a measure of arterial stiffness and it is considered that aortic PWV (Ao-PWV) is the most important measure.[Citation25] In contrast, augmentation index is thought to be a surrogate for the stiffness of arterioles.[Citation3] The QKD measures aortic and brachial PWV, and includes the pre-ejection period (PEP). Aortic PWV reflects arterial stiffness in the elderly owing to the marked increase in arterial PWV with age.[Citation29,Citation30] Ao-PWV is an established independent predictor of cardiovascular events and deaths, and coronary heart disease and stroke.[Citation1,Citation2,Citation23,Citation31,Citation32] It is therefore probable that a prolongation of QKD at night reflects a reduction in Ao-PWV and an important benefit of treatment over and above clinic BP reduction.[Citation32]
It was very interesting that the circadian rhythm for QKD did not reflect that of ABP. This reinforces the concept that that Ao-PWV is not synonymous with brachial BP and provides additional information. In fact the circadian rhythm of QKD was most closely aligned to that of heart rate. The inverse relationship between heart rate and arterial compliance has also been reported in the ambulatory central aortic pressure (AMCap) study.[Citation33] Cremer et al. [Citation32] have suggested that QKD may be employed, with mean brachial systolic BP, heart rate and height, to measure central systolic BP, as a reduction in central BP is associated with better outcome.[Citation33] It is possible that the combination of indapamide and perindopril produces benefits from either lowering aortic pulse wave velocity (Ao-PWV) or central pressure or both.
The AmCAP study [Citation33] also revealed that central pressure is relatively higher than brachial pressure at night. Thus, a reduction in central pressure at night may explain why nocturnal BP is so good a predictor of cardiovascular events.
The strengths of the sub-study are in the description of the circadian rhythm of QKD with control for BP, height and heart rate. The limitations of the study include the failure to provide baseline to treatment changes, the extrapolation of measurements of QKD to arterial PWV and, although a large study for an arterial compliance trial, failure to include the intended number of participants. The QKD interval consists of the PEP plus the pulse transit time (PTT). Changes in the former, rather than the latter could have explained the results. However, although PEP may be prolonged by an increase in BP, this is thought to be due to chronic hypertension increasing electromechanical delay [Citation34] which is unlikely to occur in the short term. Moreover, adjustment for BP did not alter our results and our results could only be explained by an increase in PEP whereas a decrease would be expected. Similarly, a reduction in pre-load could prolong PEP. This could occur at night as a diuretic can increase PEP.[Citation35] However, the QKD was prolonged even after adjustment for a lower BP, and the dose of indapamide employed was unlikely to produce a diuresis.
In conclusion, the marked reduction in cardiovascular events, and the rise in QKD at night in the HYVET trial suggests further investigation of the changes in the circadian rhythm of arterial compliance with indapamide, chlorthalidone, a thiazide diuretic, an ACE inhibitor, a calcium channel blocker and other comparators.
Disclosure statement
The authors report no conflicts of interest.
References
- Laurent S, Katsahian S, Fassot C, et al. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke. 2003;34:1203–1206.
- Blacher J, Safar ME, Guerin AP, et al. Aortic pulse wave velocity index and mortality in end-stage renal disease. Kidney Int. 2003;63:1852–1860.
- Kum F, Karalliedde J. Critical appraisal of the differential effects of antihypertensive agents on arterial stiffness. Integr Blood Press Control. 2010;3:63–71.
- Asmar RG, London GM, O’Rourke ME, et al. Improvements in blood pressure, arterial stiffness and wave reflections with a very low-dose perindopril/indapamide combination in hypertensive patients: a comparison with atenolol. Hypertension. 2001;38:922–926.
- Mackenzie IS, McEniery CM, Dhakam Z, et al. Comparisons of the effects of antihypertensive agents on central blood pressure and arterial stiffness in isolated hypertension. Hypertension. 2009;54:409–413.
- Dhakam Z, McEniery CM, Yasmin, et al. Atenolol and eprosartan: differential effects on central blood pressure and aortic pulse wave velocity. Am J Hypertens. 2006;19:214–219.
- Bulpitt CJ, Beckett N, Peters R, et al. Does white coat hypertension require treatment over age 80?: Results of the Hypertension in the Very Elderly Trial Ambulatory Blood Pressure Side Project. Hypertension. 2013;61:89–94.
- Gosse P, Jullien V, Lemetayer P, et al. Ambulatory measurements of the timing of Korotkoff sounds in a group of normal subjects: influence of age and height. Am J Hypertens. 1999;12:231–235.
- Pinto E, Bulpitt C, Beckett N, et al. Rationale and methodology of monitoring ambulatory blood pressure and arterial compliance in the Hypertension in the Very Elderly Trial. Blood Press Monit. 2006;11:3–8.
- Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–1898.
- Gosse P, Jullien V, Jarnier P, et al. Reduction in arterial dispensability in hypertensive patients as evaluated by ambulatory measurement of the QKD interval is correlated with concentric remodelling of the left ventricle. Am J Hypertens. 1999;12:1252–1255.
- O'Brien E, Waeber B, Parati G, et al. Blood pressure measuring devices: recommendations of the European Society of Hypertension. BMJ. 2001;322:531–536.
- Gosse P, Guillo P, Ascher G, et al. Assessment of arterial distensibility by monitoring the timing of Korotkoff sounds. Am J Hypertens. 1994;7:228–233.
- Gosse P, Braunstein C, Clementy J. Beyond blood pressure measurements: monitoring of the appearance of Korotkoff sounds. Blood Press Monit. 1996;1:193–196.
- Hansen TW, Li Y, Boggia J, et al. Predictive role of the night-time blood pressure. Hypertension. 2011;57:3–10.
- Kanno A, Kikuya M, Asayama K, et al. Night-time blood pressure is associated with the development of chronic kidney disease in a general population: the Ohasama study. J Hypertens. 2013;31:2410–2417.
- Cuspidi C, Facchetti R, Bombelli M, et al. Night-time blood pressure and new-onset left ventricular hypertrophy: findings from the Pamela population. Hypertension. 2013;62:78–84.
- Fagard RH, Celis H, Thijs L, et al. Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension. 2008;51:55–61.
- Ben-Dov IZ, Kark JD, Ben-Ishay D, et al. Predictors of all-cause mortality in clinical ambulatory monitoring: unique aspects of blood pressure during sleep. Hypertension. 2007;49:1235–1241.
- Dolan E, Stanton A, Thijs L, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension. 2005;46:156–161.
- Cuspidi C, Grassi G. Night-time blood pressure and new onset kidney disease. J Hypertens. 2013;31:2339–2341.
- Mallareddy M, Parikh CR, Peixoto AJ. Effect of angiotensin-converting enzyme inhibitors on arterial stiffness in hypertension: systematic review and meta-analysis. J Clin Hypertens (Greenwich). 2006;8:398–403.
- Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605.
- Williams B, Lacy PS, Thom SM, et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;7:1213–1225.
- Laurent S, Boutouyrie P. Recent advances in arterial stiffness and wave reflection in human hypertension. Hypertension. 2007;49:1202–1206.
- Stehouwer CD, Henry RM, Ferreira I. Arterial stiffness in diabetes and the metabolic syndrome: a pathway to cardiovascular disease. Diabetologia. 2008;51:527–539.
- Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–943.
- Luft FC. Angiotensin, inflammation, hypertension, and cardiovascular disease. Curr Hypertens Rep. 2001;3:61–67.
- O'Rourke MF, Nichols WW, Safar ME. Brachial and central arterial pressure. Hypertension. 2006;48:e1.
- McEniery CM, Yasmin, Hall IR, et al. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol. 2005;46:1753–1760.
- Safar ME, Levy BI, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 2003;107:2864–2869.
- Cremer A, Codjo L, Butlin M, et al. Determination of central blood pressure by a noninvasive method (brachial blood pressure and QKD interval): a noninvasive validation. J Hypertens. 2013;31:1847–1852.
- Williams B, Lacy PS, Baschiera F, et al. Novel description of the 24-hour circadian rhythms of brachial versus central aortic blood pressure and the impact of blood pressure treatment in a randomized controlled clinical trial: the Ambulatory Central Aortic Pressure (AmCAP) study. Hypertension. 2013;61:1168–1176.
- Tarazi RC, Frohlich ED, Dustan HP. Left atrial abnormality and ventricular preejection period in hypertension. Dis Chest. 1969;55:214–218.
- Buch J, Egeblad H, Hansen PB, et al. Correlation between changes in systolic time intervals and left ventricular end-diastolic diameter after preload reduction. Non-invasive monitoring of pharmacological intervention. Br Heart J. 1980;44:668–671.
Appendix
HYVET Principal Investigation: C.J.Bulpitt Co-investigation: A.E.Fletcher
HYVET Committees: Streering Committee: T. McCormack, J.Potter, B.G Extremera, P. Sever, F. Forette, D. Dumitrascu, C. Swift, J. Tuomilehto, J. Coope (retired in 2001), C. Nachev (deceased); Data Monitoring Committee: J.A.Staessen, L Thijs, R. Clarke, K. Narkiewicz; End Points Committee: C. Davidson (retired in 2003), J. Duggan, G. Leonetti, N. Gainsborough, M.C. De Vernejoul, J.Wang, V. Stoyanovsky; Ethics Committee: R. Fagard, J. Grimley Evans, B. Williams; ABPM Sub-Study Committee: C. Rajkumar, J.A. Staessen, C.J. Bulpitt
Centres participating in the ABP side project were:
Bulgaria: V. Gergova; Sofia: C. Nachev (deceased); Sofia: V. Stoyanovsky: Sofia.
China: J. Wang; Shanghai: S. Wang; Beijing: GE Yaun; Beijing; W. Zhang; Shanghai:
Finland: H. Litmanen; Kuopio: R. Antikainen: Oulu:
New Zealand: C. Anderson: Auckland
Romania: M. Comsa; Fagaras: D. Dumitrascu; Cluj: D. Jianu; Bucharest:
Russia: S. Negdogoda; Volgograd: Y. Nikitin; Novosibirsk:
United Kingdom: C. Rajkumar; London: (Principle Investigator for the ABPM sub-study)
E. Pinto coordinated the ABPM side project and W. Banya was responsible for data monitoring and preliminary analyses.