Abstract
Patients with rheumatoid arthritis (RA) and ankylosing spondylitis (AS) have increased cardiovascular (CV) morbidity and mortality. Arterial stiffness is an independent predictor of CV events. The aim of the study was to assess arterial stiffness and inflammatory markers in patients with short duration chronic arthritis. We assessed carotid-femoral pulse wave velocity (PWV), augmentation index (AIx), traditional CV risk factors and inflammatory and endothelial markers in 71 chronic arthritis patients (RA and AS) and in 29 healthy controls. We did not find differences in PWV (for RA, AS and controls, respectively: 10 [8.8–10.9] versus 10.7 [9.1–11.8] versus 9.2 [8.3–11.4] m/s; p = .14) and AIx (for RA, AS and controls, respectively: 24.3 ± 11.5 versus 5.7 ± 12.4 versus 10 ± 12.8%; p = .22). Both groups of arthritis patients had active disease with significantly elevated inflammatory markers compared to controls. There were no correlations between endothelial and inflammatory markers and parameters of arterial stiffness in arthritis patients. When analyzing arthritis patients according to median of PVW, there were no significant differences in inflammatory and endothelial markers. We found that in patients with short duration active RA and AS arterial stiffness was not increased and furthermore, there was no association between markers of systemic inflammation and arterial stiffness.
Introduction
Patients with inflammatory arthritis, as well as those with other autoimmune inflammatory diseases, have increased cardiovascular (CV) risk in comparison to the general population.[Citation1–3] Furthermore, rapid progression of atherosclerosis and increased CV mortality and morbidity are not fully explained by traditional CV risk factors.[Citation4] Increased expression of adhesion molecules, elevated level of adipocytokines or genetic factors linked to chronic inflammation may contribute to accelerated development of atherosclerosis.[Citation5–7] Some of these abnormalities have been observed in rheumatoid arthritis (RA) patients even in early stages of the disease.[Citation8] Whereas endothelial functional impairment is acknowledged in established RA patients, data about endothelial function in early RA are still scarce or inconsistent. The results of number of studies demonstrated increased common carotid intima-media thickness (IMT) and rapid progression of plaque in RA patients in comparison to the healthy controls,[Citation9] while some studies failed to show differences in terms of IMT between RA and control patients.[Citation10] The attenuated endothelial response has been observed in untreated patients with recent onset of RA [Citation11]; however, some studies demonstrated preserved endothelium-dependent vasodilatation [Citation9] or skin microvascular function.[Citation12] Furthermore, some authors presented increased arterial stiffness assessed by pulse wave velocity (PWV) in early RA patients [Citation13–15] and baseline C-reactive protein (CRP) was associated with increased PWV in 15 years’ observational study by Provan et al. [Citation16]. In meta-analysis by Ambrosino et al. in terms of data deficiency in RA subjects, it was difficult to evaluate other indices of arterial stiffness such as augmentation index (AIx) in early RA patients.[Citation4]
Ankylosing spondylitis (AS) patients have elevated CV morbidity and mortality rates when compared to general population.[Citation2,Citation3] The results of studies on endothelial and vascular function in AS patients are contradictory. Some studies have shown increased IMT,[Citation17,Citation18] impaired endothelium-dependent vasodilatation [Citation18,Citation19] or increased aortic stiffness,[Citation18,Citation20] whereas the other results did not confirm this findings.[Citation17]
In summary, both AS and RA, in spite of different pathogenesis and specific clinical symptoms are chronic inflammatory diseases and elevated CV risk is common for both arthritis patients. AIx and PWV have been used to assess subclinical organ damage and presented positive prognostic value in predicting CV events,[Citation21,Citation22] but data on arterial stiffness in these patients are scarce and conflicting.
The aim of this study was to assess the arterial stiffness and the relationship between arterial stiffness and inflammatory markers in patient with RA and AS.
Material and methods
The study included 100 patients, 71 chronic arthritis patients (26 with RA and 45 with AS) and 29 healthy controls. The exclusion criteria were: uncontrolled hypertension, treatment with disease modifying anti-rheumatic drugs (DMARDs), acute infection within 2 weeks preceding the study, evidence of atherosclerotic CV disease (history of myocardial infarction, stroke/transitional ischemic attack, coronary artery disease), diabetes, renal failure and history of neoplasm within 5 years after termination of the treatment.
Patients were recruited from the Outpatient Rheumatology Clinic of the Department of Internal Medicine of the University Hospital in Krakow as described previously.[Citation8,Citation23,Citation24] Diagnosis was established according to the revised 1987 American College of Rheumatology Criteria [Citation25] or the modified New York criteria [Citation26] for RA and anyklosing spondylitis, respectively.
The study protocol was approved by the Bioethical Committee of Jagiellonian University. Each patient had given written informed consent before the study inclusion. Patients were asked to refrain from eating and smoking for at least 12 h before the evaluation. The patients’ evaluation included physical examination, anthropometric measurements (weight, height, waist and hip circumference, body mass index, waist to hip ratio), medical history of medications, smoking status and family history of premature CV disease. The disease activity was assessed with DAS28 (Disease Activity Score) for RA patients and BASDAI (The Bath Ankylosing Spondylitis Disease Activity Index) for AS. Three blood pressure measurements were taken on left arm after 5 min resting in a sitting position. The averaged value of the last two measurements was calculated.
Noninvasive haemodynamic indices of arterial stiffness (PWV and Alx) were assessed. Arterial stiffness and wave reflections-radial artery waveforms were recorded using tonometry and transferred to construct the corresponding waveform in the central aorta using SphygmoCor (AtCor Medical, West Ryde, Australia). The effects of arterial stiffness and arterial wave reflection on the central aortic waveform were calculated as: aortic pulse-wave augmentation (AG) – the difference between the second (P2) and the first (P1) systolic peaks of the central arterial waveform, the Alx – the difference between the second (P2) and the first (P1) systolic peaks, divided by pulse pressure expressed as a percentage (AG/PP) or the second systolic peak (P2) divided by the pressure to the first systolic peak (P1) of the central arterial waveform (P2/P1). Pulse wave was analysed through central pulse pressure and central systolic pressure. Carotid-femoral PWV was assessed using Complior (Complior, Colson, Garges-les-Gonesse, France). Two pressure sensitive transducers were placed directly on the skin above femoral and carotid artery track. The average of 10 consecutive measurements was considered for analysis followed by elimination of two extreme values (the minimum and maximum ones). PWV was calculated automatically as a proportion of total distance between the carotid and femoral sites and a wave transition’s time.
Blood samples were taken from the left antecubical vein to evaluate the interleukin-6 (IL-6), interleukin 18 (IL-18), tumor necrosis factor α (TNF-α), high sensitivity C-reactive protein (hsCRP), fibrinogen, pentraxin 3 (PTX-3), soluble form of intracellular adhesion molecule-1 (sICAM), soluble form of vascular cell adhesion molecules (sVCAM), soluble form of E-selectin (sE-selectin), von Willebrand factor (vWF), tissue plasminogen activator (tPA) were measured using immunonephelometry or enzyme-linked immunosorbent assay (ELISA). Plasma fibrinogen was estimated with a BCS Coagulation Analyzer (Siemens Healthcare Diagnostics Inc., Erlangen, Germany). For biochemical parameters (serum fasting glucose, total cholesterol, triglycerides, LDL-cholesterol, HDL-cholesterol, creatinine) measured with Hitachi 917 analyser (Roche Diagnostics, F. Hoffmann-La Roche AG, Basel, Switzerland) were assessed using standardized laboratory technics.
Statistical analysis
Statistical analysis was performed using Statistica 12 software (StatSoft Inc., Tulusa, OK). The accordance with normal distribution was tested by Shapiro–Wilk test and the uniformity of variance by Levene’s test. To assess differences between the groups, we used one-way ANOVA and post-hoc Bonferroni test or Mann–Whitney U test, Student’s t-test and χ2 test according to the distribution pattern and groups quantity. General linear models (GLM) or covariation test analysis were applied to test adjusted gender and age p value. P value equal or less than 0.05 was considered statistically significant. Correlation between PWV, AIx, peripheral and central systolic and diastolic blood pressure value as well as markers of inflammation was tested by Spearman’s correlation test.
Results
Mean age of the patients was 40 years (SD ±9.7), 33 years (SD ±6.6) and 32 years (SD ±7.6) in RA patients, AS patients and healthy controls, respectively. The characteristics of patients and the control group are provided in . The median duration of symptoms in RA patients was 12 [4–22, 5] months and in AS patients 6 [4–10] years. RA patients were predominantly women and were older than both control and AS subjects. Furthermore, groups differed in terms of HDL cholesterol, creatinine and HbA1C level. The disease activity was moderate (DAS28:4.4 ± 1.5) for RA and in AS - BASDAI score was 4.2 (±2.0), indicating active disease.
Table 1. Characteristic of the patients in the study.
In RA and AS patients, we observed increased ESR, hsCRP, IL-6, fibrinogen and TNF-α, whereas after standardization for age and gender the TNF-α value did not differ between the groups. Some endothelial markers were significantly increased in both RA and AS groups (PTX-3, vWF, sVCAM), the highest level of sICAM was found in AS. The healthy control and RA patients did not differ in terms of IL-18 and tPA (). After adjustment for age and gender, there were no statistically significant differences between patients in arterial stiffness measured with PWV (for RA, AS and controls, respectively: 10 [8.8–10.9] versus 10.7 [9.1–11.8] versus 9.2 [8.3–11.4] m/s; p = .14), as well as with AG (for RA, AS and controls, respectively: 7.8 ± 6.3 versus 2.1 ± 3.6 versus 3.4 ± 3.8 mmHg; p = .69) and AIx (AG/PP) (for RA, AS and controls, respectively: 24.3 ± 11.5 versus 5.7 ± 12.4 versus 10 ± 12.8%; p = .22), (P2/P1) (for RA, AS and controls, respectively 133.7 ± 22.7 versus 109.3 ± 14.5 versus 115.5 ± 16.6 versus 115.5 ± 16.6%; p = .5) (). Detailed parameters of arterial stiffness are available in in Online Data Supplement.
Figure 1. Blood pressure and arterial stiffness in RA and AS patients and in the control group. A. Blood pressure and mean arterial pressure (mmHg); B. Central blood pressure and central pulse pressure (mmHg); C. Pulse wave velocity (m/s); D. Aortic pulse wave augmentation (AG); E. AIx augmentation indexes. There were no statistically significant differences between the groups after age and sex standardization (p > .05).
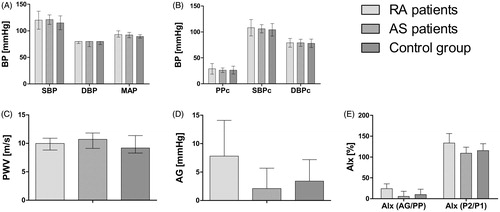
Table 2. Inflammatory markers in RA and AS patients and in the control group.
We analysed RA and AS patients together as a one group and divided the group into two categories according to median PWV. Patients with median value of PWV equal or above 10.1 m/s had elevated MAP (93 [90–100] versus 89 [85–93] mmHg; p = .02) and PPc (28 [24–34] versus 25 [22–28] mmHg; p = .046), increased AG (4 [1–8] versus 2 [0.5–4] mmHg; p = .02) but Alx(AG/PP) (14.5 ± 16.2 versus 9.3 ± 13.1%; p = .18), Alx (P2/P1) (121.1 ± 22.6 versus 113.2 ± 18.3% p = .15) were similar in both groups ( available in Online Data Supplement). The groups did not differ in any measured endothelial and inflammatory markers (data not shown). There were no correlations between inflammatory or endothelial markers and vascular function in arthritis patients.
Discussion
Patients with RA have increased CV mortality and morbidity in comparison to general population and it cannot be fully explained by traditional CV risk factors. There is evidence linking accelerated atherogenesis with systemic inflammation.[Citation27] Circulating inflammatory cytokines exert its functions in numerous tissues and organs causing insulin resistance, dyslipidemia, endothelial dysfunction, prothrombotic effect and pro-oxidative stress contributing to accelerated atherosclerosis. Aortic PWV may be a surrogate end point for CV disease and together with central haemodynamic indices it is a strong predictor of future CV events.[Citation21,Citation22] Thus, in this work we attempted to assess arterial stiffness in DMARD naive inflammatory arthritis patients in comparison to healthy subjects. Our results did not reveal any statistically significant differences between groups after adjustment for age and gender in terms of aortic PWV and central haemodynamics. These findings were consistent with some previous studies which showed no differences in arterial stiffness indices in AS in comparison to control group [Citation17] or between chronic inflammatory rheumatic disease groups.[Citation14] Sandoo et al. [Citation28] showed that PWV was elevated in several cross-sectional studies, nevertheless most of analysed studies described patients with established RA with exception to Avalos et al. [Citation15], who did not demonstrate any differences in brachial PWV in early RA. In contrary to these and our data, some studies have reported elevated aortic PWV value even in early RA.[Citation4] Our results depicting PWV and AIx are not in concordance with results of meta-analysis performed by Ambrosino et al. [Citation4]. However, the authors of this meta-analysis showed a publication bias concerning aortic PWV, what may to certain extent explain the discrepancy between our results and results of this meta-analysis. Moreover, in this meta-analysis, the AIx was shown to be higher in RA but that observation was based on the results of only one study which was done in early RA patients.[Citation29] Furthermore, there may be an increased CV risk even before or shortly after the onset of RA. The results of some studies indicated that CV risk may accumulate during years of disease duration, whereas there were no evident CV changes in early stage.[Citation30] On the other hand, several reports indicate that disease duration does not independently affect the risk of CVD but rather the overall time with persistent high disease activity contribute the most.[Citation31] Treatment with TNF-α antagonists may probably partially reverse or intermit vascular changes whereas it was not certain if the effect is specific for this drug or related to decrease in inflammation.[Citation32]
We have divided the inflammatory arthritis group according to the aortic PWV median value. We did not find significant differences in any inflammatory or endothelial markers despite of differences in peripheral and central blood pressure as well as in AG. We did not find any relationship between indices of arterial stiffness and inflammatory markers in combined inflammatory arthritis group, which is consistent with previous reports [Citation28,Citation33] assessing micro- and macrovascular endothelial function in correlation to inflammation.
In our study, patients and healthy controls did not present any differences in terms of classical CV disease risk factors with exception of HDL cholesterol. The lowest level of HDL was found in AS patients. That finding might be explained by the fact that in active untreated inflammatory disease the lipid level may be reduced and increase during treatment, whereas it may not represent increased CV risk.[Citation34] Lower HDL cholesterol level presumably reflects increased inflammatory state and our results are in agreement with results of previously reported studies.
Limitations of the study
Nonetheless, some limitations must be taken into consideration when interpreting the results of our study. First, some of arthritis patients were treated with glucocorticoids (38.4% in RA and 5.2% in AS) or non-steroidal anti-inflammatory drugs (62% in RA and 92% in AS) which may influence endothelial function. Second, there were significant differences in age and gender between the studied groups, so the results have been adjusted for these factors. Third, heart rate may influence Alx, but these data were not recorded in our study. Finally, the patients have not had 24-h blood pressure monitoring study, which would have allowed to estimate the dynamic relationship between SBP and DBP during the course of the day.
Conclusions
We found that in patients with short duration active RA and AS arterial stiffness was not increased. Additionally, there was no association between markers of systemic inflammation and arterial stiffness.
Supplemental_online_material_1_.docx
Download MS Word (26.6 KB)Disclosure statement
The authors report no conflicts of interests.
References
- Kremers HM, Crowson CS, Therneau TM, et al. High ten-year risk of cardiovascular disease in newly diagnosed rheumatoid arthritis patients: a population-based cohort study. Arthritis Rheum. 2008;58:2268–2274.
- Mathieu S, Motreff P, Soubrier M. Spondyloarthropathies: an independent cardiovascular risk factor? Joint Bone Spine. 2010;77:542–545.
- Han C, Robinson DW Jr, Hackett MV, et al. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol. 2006;33:2167–2172.
- Ambrosino P, Tasso M, Lupoli R, et al. Non-invasive assessment of arterial stiffness in patients with rheumatoid arthritis: a systematic review and meta-analysis of literature studies. Ann Med. 2015;47:457–467.
- Soltesz P, Kerekes G, Der H, et al. Comparative assessment of vascular function in autoimmune rheumatic diseases: considerations of prevention and treatment. Autoimmun Rev. 2011;10:416–425.
- Sandoo A, Chanchlani N, Hodson J, et al. The relationship between cardiovascular disease risk prediction scores and vascular function and morphology in rheumatoid arthritis. Clin Exp Rheumatol. 2014;32:914–921.
- Montecucco F, Mach F. Common inflammatory mediators orchestrate pathophysiological processes in rheumatoid arthritis and atherosclerosis. Rheumatology. 2009;48:11–22.
- Klimek E, Skalska A, Kwasny-Krochin B, et al. Differential associations of inflammatory and endothelial biomarkers with disease activity in rheumatoid arthritis of short duration. Mediators Inflamm. 2014;2014:681635.
- Ambrosino P, Lupoli R, Di Minno A, et al. Subclinical atherosclerosis in patients with rheumatoid arthritis. A meta-analysis of literature studies. Thromb Haemost. 2015;113:916–930.
- Sodergren A, Karp K, Boman K, et al. Atherosclerosis in early rheumatoid arthritis: very early endothelial activation and rapid progression of intima media thickness. Arthritis Res Ther. 2010;12:R158.
- Chatterjee Adhikari M, Guin A, Chakraborty S, et al. Subclinical atherosclerosis and endothelial dysfunction in patients with early rheumatoid arthritis as evidenced by measurement of carotid intima-media thickness and flow-mediated vasodilatation: an observational study. Semin Arthritis Rheum. 2012;41:669–675.
- van Eijk IC, Serne EH, Dijkmans BA, et al. Microvascular function is preserved in newly diagnosed rheumatoid arthritis and low systemic inflammatory activity. Clin Rheumatol. 2011;30:1113–1118.
- Turkyilmaz AK, Devrimsel G, Kirbas A, et al. Relationship between pulse wave velocity and serum YKL-40 level in patients with early rheumatoid arthritis. Rheumatol Int. 2013;33:2751–2756.
- Kocabay G, Hasdemir H, Yildiz M. Evaluation of pulse wave velocity in systemic lupus erythematosus, rheumatoid arthritis and Behçet’s disease. J Cardiol. 2012;59:72–77.
- Avalos I, Chung CP, Oeser A, et al. Increased augmentation index in rheumatoid arthritis and its relationship to coronary artery atherosclerosis. J Rheumatol. 2007;34:2388–2394.
- Provan SA, Angel K, Semb AG, et al. Early prediction of increased arterial stiffness in patients with chronic inflammation: a 15-year follow-up study of 108 patients with rheumatoid arthritis. J Rheumatol. 2011;38:606–612.
- Mathieu S, Joly H, Baron G, et al. Trend towards increased arterial stiffness or intima-media thickness in ankylosing spondylitis patients without clinically evident cardiovascular disease. Rheumatology. 2008;47:1203–1207.
- Bodnar N, Kerekes G, Seres I, et al. Assessment of subclinical vascular disease associated with ankylosing spondylitis. J Rheumatol. 2011;38:723–729.
- van Eijk IC, Peters MJ, Serne EH, et al. Microvascular function is impaired in ankylosing spondylitis and improves after tumour necrosis factor alpha blockade. Ann Rheum Dis. 2009;68:362–366.
- Berg IJ, van der Heijde D, Dagfinrud H, et al. Disease activity in ankylosing spondylitis and associations to markers of vascular pathology and traditional cardiovascular disease risk factors: a cross-sectional study. J Rheumatol. 2015;42:645–653.
- Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327.
- Vlachopoulos C, Aznaouridis K, O’Rourke MF, et al. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J. 2010;31:1865–1871.
- Klimek E, Mikolajczyk T, Sulicka J, et al. Blood monocyte subsets and selected cardiovascular risk markers in rheumatoid arthritis of short duration in relation to disease activity. Biomed Res Int. 2014;2014:736853.
- Surdacki A, Sulicka J, Korkosz M, et al. Blood monocyte heterogeneity and markers of endothelial activation in ankylosing spondylitis. J Rheumatol. 2014;41:481–489.
- Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324.
- van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361–368.
- Di Minno MN, Iervolino S, Lupoli R, et al. Cardiovascular risk in rheumatic patients: the link between inflammation and atherothrombosis. Semin Thromb Hemost. 2012;38:497–505.
- Sandoo A, Veldhuijzen van Zanten JJ, Metsios GS, et al. Vascular function and morphology in rheumatoid arthritis: a systematic review. Rheumatology. 2011;50:2125–2139.
- Lo Gullo A, Mandraffino G, Imbalzano E, et al. Toll-like receptor 3 and interleukin 1β expression in CD34+ cells from patients with rheumatoid arthritis: association with inflammation and vascular involvement. Clin Exp Rheumatol. 2014;32:922–929.
- Chung CP, Oeser A, Raggi P, et al. Increased coronary-artery atherosclerosis in rheumatoid arthritis: relationship to disease duration and cardiovascular risk factors. Arthritis Rheum. 2005;52:3045–3053.
- Arts EE, Fransen J, den Broeder AA, et al. The effect of disease duration and disease activity on the risk of cardiovascular disease in rheumatoid arthritis patients. Ann Rheum Dis. 2015;74:998–1003.
- Tam LS, Kitas GD, Gonzalez-Gay MA. Can suppression of inflammation by anti-TNF prevent progression of subclinical atherosclerosis in inflammatory arthritis? Rheumatology. 2014;53:1108–1119.
- Sandoo A, Kitas GD, Carroll D, et al. The role of inflammation and cardiovascular disease risk on microvascular and macrovascular endothelial function in patients with rheumatoid arthritis: a cross-sectional and longitudinal study. Arthritis Res Ther. 2012;14:R117.
- Choy E, Sattar N. Interpreting lipid levels in the context of high-grade inflammatory states with a focus on rheumatoid arthritis: a challenge to conventional cardiovascular risk actions. Ann Rheum Dis. 2009;68:460–469.
