Abstract
We tested the prognostic impact of a marker of arterial stiffness, pulse pressure/stroke volume index (PP/SVi), in patients with hypertension and left ventricular (LV) hypertrophy. We used data from 866 patients randomized to losartan or atenolol-based antihypertensive treatment, over a median of 4.8 years, in the Losartan Intervention For Endpoint reduction in hypertension (LIFE) study. The association of PP/SVi with outcomes was tested in Cox regression analyses and reported as hazard ratio (HR) and 95% confidence intervals (CI). In multivariate regression, reduction of PP/SVi was independently associated with male gender, reduction in systolic blood pressure (BP) and relative wall thickness and with an increase in left ventricular ejection fraction (all p < .05). After adjusting for confounders, higher baseline PP/SVi predicted a 38% higher hazard of combined major fatal and non-fatal cardiovascular events (95% CI 1.04–1.84), and higher hazard of cardiovascular mortality (HR 2.35 (95% CI 1.59–3.48) and stroke (HR 1.45 (95% CI 1.06–1.99) (all p < .05). Higher PP/SVi also predicts higher rate of hospitalization for HF (HR 2.15 (95% CI 1.48–3.12) and a 52% higher hazard of all-cause mortality (95% CI 1.10–2.09) (both p < .05). In hypertensive patients with electrocardiographic LV hypertrophy, higher PP/SVi was associated with increased cardiovascular morbidity and mortality.
Introduction
In hypertension, increased arterial stiffness is associated with abnormal left ventricular (LV) geometry, diastolic dysfunction, abnormal segmental relaxation and decreased longitudinal systolic deformation.[Citation1–4] Increased arterial stiffness is also associated with other markers of subclinical CV disease and predicts CV morbidity and mortality better than clinical blood pressure (BP) and independently of LV hypertrophy.[Citation5–8]
The Losartan Intervention For Endpoint reduction in hypertension (LIFE) study increased pulse pressure (PP) as a marker of arterial stiffness was associated with increased risk of atrial fibrillation as well as other cardiovascular events.[Citation9]
Arterial stiffness may be defined as the relationship between changes in arterial pressure per unit of change in arterial volume, and pulse pressure/stroke volume index ratio (PP/SVi) by echocardiography is considered an acceptable and reproducible method for the estimation of arterial stiffness, as documented in previous studies.[Citation6,Citation10] Higher arterial stiffness has been studied both in women and in men with hypertension by different methods and modalities including pulse pressure, PP/SVi ratio, aortic characteristic impedance and pulse wave velocity.[Citation11–13] However, the prognostic importance of PP/SVi has not been reported from a prospective study in hypertensive subjects with LV hypertrophy. This was the aim of the present pre-specified analysis.
Methods
Study population
The present pre-specified analysis was undertaken in the echocardiography sub-study of the Losartan Intervention For Endpoint reduction in hypertension (LIFE) study which included 960 of the 9193 patients of the main LIFE study.[Citation14,Citation15] The LIFE study randomized hypertensive patients aged 55–80 years with baseline-seated systolic BP of 160–200 or diastolic BP of 95–115 mmHg and electrocardiographic (ECG) LV hypertrophy, to >4 years double-blind losartan-based and atenolol-based antihypertensive treatment. Characteristics and main results of the LIFE echocardiography sub-study population have been previously reported.[Citation16,Citation17]
Among the 960 patients included in the LIFE echocardiography sub-study, the present analysis included 866 patients in whom PP/SVi could be assessed at baseline and at least one follow-up echocardiogram.
BP, diabetes and albuminuria
Seated BP was measured in triplets by arm-cuff sphygmomanometer at clinical study visits, and the average of the two last measurements was taken as the clinic BP. Pulse pressure (PP) was calculated as the difference between systolic and diastolic BP. Patients were classified as having isolated systolic hypertension (ISH) if systolic BP ≥160 mmHg and diastolic BP <90 mmHg.[Citation14] Estimated glomerular filtration rate (eGFR) was calculated from MDRD simplified formula.[Citation18] Albuminuria was assessed in spot morning urine at baseline and considered present if albumin/creatinine ratio exceeded 3.5 mg/mmol.[Citation19] Diabetes mellitus was diagnosed by the 1985 WHO criteria or use of hypoglycemia medication.[Citation20]
Echocardiography
The LIFE study echocardiographic protocol and methods have been previously published.[Citation16] In short, echocardiograms were performed at baseline and annually thereafter, and sent for expert interpretation to the Reading Center at the Weill Cornell Medical Center, New York, United States of America. LV ejection fraction was calculated by the Teichholz method and LV mass using an autopsy validated formula.[Citation21] LV hypertrophy was defined as LV mass/height2.7 (LVMi) > 49.2g/m2.7 in men and >46.7g/m2.7 in women.[Citation22] Relative wall thickness (RWT) was calculated at end-diastole as the ratio between posterior wall thickness (PWT) * 2/LV internal dimension and considered increased if ≥0.43.[Citation23] LV wall mechanics were assessed by midwall shortening (MWS) adjusted for circumferential end-systolic stress.[Citation24] Stroke volume (SV) was assessed by Doppler as previously described.[Citation25] Arterial stiffness was estimated from PP/SV indexed for height in the allometric power of 2.04 (PP/SVi).[Citation7,Citation26] Aortic and mitral regurgitation were assessed by colour Doppler using previously described four-point grading systems.[Citation27,Citation28]
Study end-points
The pre-specified primary end-point in the study was a composite of the first occurrence of major cardiovascular events (cardiovascular death, non-fatal stroke and non-fatal myocardial infarction). Pre-specified secondary specific end-points were cardiovascular death, fatal and non-fatal stroke, and fatal and non-fatal myocardial infarction, and pre-specified tertiary end-points included hospitalization for heart failure (HF) and all-cause mortality. All endpoints were adjudicated by an independent End-Point Committee.[Citation15]
Statistical analysis
Data management and analysis were performed using IBM SPSS 22 software (IBM, Armonk, NY). Data are presented as mean ±1 SD for continuous variables and as percentages for categorical variables. Baseline and follow-up values were compared by the analysis of variance for repeated measurement. Linear regression analysis was used to identify correlates of change in PP/SVi during follow-up. The associations of baseline and in-treatment PP/SVi with pre-specified study endpoints were assessed by Cox and time-varying Cox models. Multivariable model for the primary study end-point was adjusted for baseline systolic BP, PP and LV mass, sex and treatment allocation. Multivariable models for secondary and tertiary end-points were adjusted only for baseline systolic BP and treatment allocation due to the lower number of events.
The last follow-up study visit was defined as the last one performed before a primary event occurred in the patients who experienced events, and as the last study visit in patients without events. Two-tailed p < .05 was considered statistical significant in all analyses.
Results
Compared with participants in the main LIFE trial who did not participate in the echocardiography sub-study, the present study population had lower systolic BP and PP at baseline and included higher proportion of Africans American subjects, but lower proportion of women and subject with obesity ().
Table 1. Baseline characteristics of the current study population compare to the main LIFE study population not included in the echocardiography sub-study.
During follow-up, systolic and diastolic BP (−38 mmHg and −18 mmHg, respectively), RWT, EF, LV mass index and PP/SVi decreased while MWS and SVi increased () (all p < .05).
Table 2. Echocardiographic findings at baseline and last study visit.
Covariates of PP/SVi
At baseline, higher PP/SVi correlated with older age, male gender, higher systolic BP and RWT, but with lower BMI, eGFR, LV ejection fraction and MWS (all p < .05). In multivariable linear regression, reduction of PP/SVi was independently associated with male gender, reduction in systolic BP, RWT and with increase in EF (all p < .05) ().
Table 3. Covariables of PP/SVi reduction in the current study population. Multivariable linear regression.
Association of PP/SVi with outcome
In Cox regression analysis, higher PP/SVi at baseline was associated with higher rate of major cardiovascular events both in women and in men (both p < 0.05) (). In univariate Cox regression analysis, higher baseline PP/SVi predicts a 31% higher hazard of combined major cardiovascular events, the primary study end-point, 110% and 42% higher hazard rates of cardiovascular mortality and stroke, respectively (all p < .05) (). Higher PP/SVi at baseline also predicted 120% higher rate of hospitalization for heart failure and 35% higher rate of all-cause mortality (all p < .05) (). After adjusting for baseline systolic BP, PP and LV mass, sex and treatment allocation in multivariable Cox regression, higher baseline PP/SVi predicted a 38% higher hazard of combined major fatal and non-fatal cardiovascular events (p < .05) (). Furthermore higher PP/SVi at baseline predict higher hazard of cardiovascular mortality and stroke, a 52% higher hazard of all-cause mortality and hospitalization for HF, independent of treatment allocation and baseline systolic BP (all p < .05) ().
Figure 1. Association of baseline PP/SVi with hazard for major cardiovascular events in men and women.
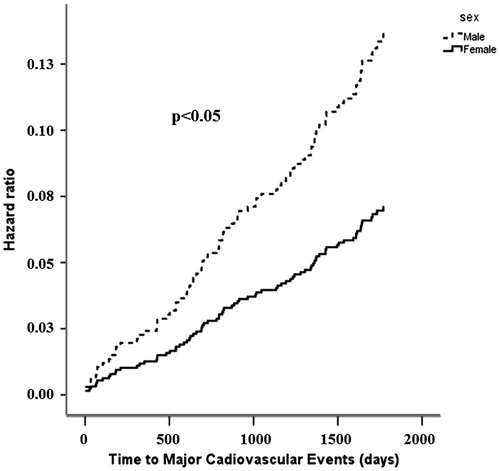
Table 4. Relative unadjusted and adjusted hazard rate for primary cardiovascular end-point, cardiovascular mortality, stroke, myocardial infarction, hospitalization for heart failure and total mortality for baseline PP/SVi.
In univariate time-varying Cox regression analysis, higher in-treatment PP/SVi was particularly associated with higher rate of hospitalization for HF (HR 1.96 [95% CI 1.37–2.81], p = .0001), while no associations with the primary or secondary end-points were found (data not shown). After adjusting for in-treatment systolic BP and treatment allocation, higher in-treatment PP/SVi retained its association with higher rate of hospitalization for HF (HR 2.48 [95% CI 1.70–3.61], p = .0001).
Discussion
The present study tested the association of PP/SVi, a measure of arterial stiffness based upon a 2-element Windkessel model, with cardiovascular morbidity and mortality in hypertensive patients with ECG signs of LV hypertrophy participating in the LIFE study. As demonstrated, higher PP/SVi at baseline was associated with increased cardiovascular morbidity and mortality, and a 2-fold increased hazard rate for hospitalization for HF was demonstrated, despite effective antihypertensive treatment.
The prognostic impact of PP/SVi in hypertensive patients was previously reported by Fagard et al. using invasive hemodynamic measurements, demonstrating that higher PP/SVi was a significant and independent predictor of CV events and all cause-mortality.[Citation5] The present results add to this by demonstrating that also during systematic antihypertensive treatment in subjects with LV hypertrophy, higher PP/SVi was associated with increased CV morbidity and mortality independent of prognostic beneficial reduction in systolic BP and LV mass, as previously reported from the LIFE study.[Citation16,Citation17]
In the population-based Rotterdam study, arterial stiffness, measured by aortic pulse wave velocity, predicted incident coronary artery disease and stroke independent of well-known cardiovascular risk factors.[Citation29] Even though we used a less sophisticated measure of arterial stiffness, our results confirm the impaired prognosis associated with higher arterial stiffness in a population with longstanding hypertension and target organ damage.
It has been reported previously by Palmieri et al. [Citation3] that increased PP/SVi at baseline in the LIFE study was associated with the presence of concentric LV geometry and with diastolic LV abnormalities suggestive of increased diastolic stiffness. Palmieri et al. [Citation30] also noted that less reduction in PP/SVi during the first treatment year was particularly associated with reduction in relative wall thickness, while no association with reduction in LV mass was found. Furthermore, residual concentric LV geometry despite antihypertensive treatment has been demonstrated to carry the worst prognosis.[Citation31] The present results add to this by demonstrating that less PP/SVi reduction during follow up was observed in women and in patients with less reduction in systolic BP and relative wall thickness. Taken together with the previous report, these factors may help explain the association of higher PP/SVi with impaired prognosis.
A recent meta-analysis showed that the predictive value of increased arterial stiffness is larger in patients with higher cardiovascular risk.[Citation32] The present study population of hypertensive patients with ECG LV hypertrophy represent a very high-risk population, and the finding that higher PP/SVi predicted higher cardiovascular event rate even after adjustment for systolic BP and LV mass adds to previous reports.[Citation17]
Increased pulse pressure (PP) is associated with increased pulsatile load on the heart, leading to susceptibility to ischemia, diastolic dysfunction and adverse clinical events.[Citation33,Citation34] When high PP coexists together with low stroke volume, the perfusion of organs like brain, heart and kidney can be further compromised due to the increased pulsatility of blood flow to the brain and kidney and lower blood flow during diastole to the myocardium, leading to myocardial ischemia and brain and renal damage.[Citation29,Citation35,Citation36]
In conclusion, in hypertensive patients with ECG LV hypertrophy, higher PP/SVi was associated with increased cardiovascular morbidity and mortality, including hospitalization for HF independent of well-known cardiovascular risk factors like systolic BP, PP and LV mass.
Disclosure statement
C. M., M. T. L, H. M. and G. d. S. have no disclosures in relation to this manuscript.
Funding
E. G., K. W. and K. B. have all received grant support from Merck & Co, the sponsor of the LIFE study during the study conduct from 1995–2002. R.B.D. and B.D. have received grant support and served as consultants for Merck & Co.
References
- Ye Z, Coutinho T, Pellikka PA, et al. Associations of alterations in pulsatile arterial load with left ventricular longitudinal strain. Am J Hypertens. 2015;28:1325–1331.
- Pavlopoulos H, Nihoyannopoulos P. Pulse pressure/stroke volume: a surrogate index of arterial stiffness and the relation to segmental relaxation and longitudinal systolic deformation in hypertensive disease. Eur J Echocardiogr. 2009;10:519–526.
- Palmieri V, Bella JN, Roman MJ, et al. Pulse pressure/stroke index and left ventricular geometry and function: the LIFE study. J Hypertens. 2003;21:781–787.
- Mottram PM, Haluska BA, Leano R, et al. Relation of arterial stiffness to diastolic dysfunction in hypertensive heart disease. Heart. 2005;91:1551–1556.
- Fagard RH, Pardaens K, Staessen JA, et al. The pulse pressure-to-stroke index ratio predicts cardiovascular events and death in uncomplicated hypertension. J Am Coll Cardiol. 2001;38:227–231.
- de Simone G, Roman MJ, Koren MJ, et al. Stroke volume/pulse pressure ratio and cardiovascular risk in arterial hypertension. Hypertension. 1999;33:800–805.
- Casalnuovo G, Gerdts E, de Simone G, et al. Arterial stiffness is associated with carotid atherosclerosis in hypertensive patients (the Campania Salute Network). Am J Hypertens. 2012;25:739–745.
- Midtbø H, Gerdts E, Kvien TK, et al. The association of hypertension with asymptomatic cardiovascular organ damage in rheumatoid arthritis. Blood Press. 2016;25:298–304.
- Larstorp AC, Ariansen I, Gjesdal K, et al. Association of pulse pressure with new-onset atrial fibrillation in patients with hypertension and left ventricular hypertrophy: the Losartan Intervention For Endpoint (LIFE) reduction in hypertension study. Hypertension. 2012;60:347–353.
- Randall OS, Westerhof N, van den Bos GC, et al. Reliability of stroke volume to pulse pressure ratio for estimating and detecting changes in arterial compliance. J Hypertens Suppl. 1986;4:S293–S296.
- Mancusi C, Gerdts E, De Simone G, et al. Impact of isolated systolic hypertension on normalization of left ventricular structure during antihypertensive treatment (the LIFE study). Blood Press. 2014;23:206–212.
- Coutinho T, Borlaug BA, Pellikka PA, et al. Sex differences in arterial stiffness and ventricular-arterial interactions. J Am Coll Cardiol. 2013;61:96–103.
- Russo C, Jin Z, Palmieri V, et al. Arterial stiffness and wave reflection: sex differences and relationship with left ventricular diastolic function. Hypertension. 2012;60:362–368.
- Dahlöf B, Devereux RB, Julius S, et al. Characteristics of 9194 patients with left ventricular hypertrophy: the LIFE study. Losartan Intervention for Endpoint Reduction in Hypertension. Hypertension. 1998;32:989–997.
- Dahlöf B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003.
- Devereux RB, Dahlof B, Gerdts E, et al. Regression of hypertensive left ventricular hypertrophy by losartan compared with atenolol: the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial. Circulation. 2004;110:1456–1462.
- Devereux RB, Wachtell K, Gerdts E, et al. Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA. 2004;292:2350–2356.
- Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470.
- Jensen JS, Clausen P, Borch-Johnsen K, et al. Detecting microalbuminuria by urinary albumin/creatinine concentration ratio. Nephrol Dial Transplant. 1997;12:6–9.
- WHO Study Group. Diabetes mellitus (technical report series 727). Geneva: WHO; 1985.
- Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458.
- de Simone G, Izzo R, Chinali M, et al. Does information on systolic and diastolic function improve prediction of a cardiovascular event by left ventricular hypertrophy in arterial hypertension? Hypertension. 2010;56:99–104.
- de Simone G, Daniels SR, Kimball TR, et al. Evaluation of concentric left ventricular geometry in humans: evidence for age-related systematic underestimation. Hypertension. 2005;45:64–68.
- de Simone G, Devereux RB, Koren MJ, et al. Midwall left ventricular mechanics. An independent predictor of cardiovascular risk in arterial hypertension. Circulation. 1996;93:259–265.
- Devereux RB, Roman MJ, Paranicas M, et al. Relations of Doppler stroke volume and its components to left ventricular stroke volume in normotensive and hypertensive American Indians: the Strong Heart study. Am J Hypertens. 1997;10:619–628.
- de Simone G, Devereux RB, Daniels SR, et al. Stroke volume and cardiac output in normotensive children and adults. Assessment of relations with body size and impact of overweight. Circulation. 1997;95:1837–1843.
- Lebowitz NE, Bella JN, Roman MJ, et al. Prevalence and correlates of aortic regurgitation in American Indians: the Strong Heart study. J Am Coll Cardiol. 2000;36:461–467.
- Jones EC, Devereux RB, Roman MJ, et al. Prevalence and correlates of mitral regurgitation in a population-based sample (the Strong Heart Study). Am J Cardiol. 2001;87:298–304.
- Mattace-Raso FU, van der Cammen TJ, Hofman A, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam study. Circulation. 2006;113:657–663.
- Palmieri V, Bella JN, Gerdts E, et al. Change in pulse pressure/stroke index in response to sustained blood pressure reduction and its impact on left ventricular mass and geometry changes: the life study. Am J Hypertens. 2008;21:701–707.
- Gerdts E, Cramariuc D, de Simone G, et al. Impact of left ventricular geometry on prognosis in hypertensive patients with left ventricular hypertrophy (the LIFE study). Eur J Echocardiogr. 2008;9:809–815.
- Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327.
- Kingwell BA, Waddell TK, Medley TL, et al. Large artery stiffness predicts ischemic threshold in patients with coronary artery disease. J Am Coll Cardiol. 2002;40:773–779.
- Domanski MJ, Mitchell GF, Norman JE, et al. Independent prognostic information provided by sphygmomanometrically determined pulse pressure and mean arterial pressure in patients with left ventricular dysfunction. J Am Coll Cardiol. 1999;33:951–958.
- Michener KH, Mitchell GF, Noubary F, et al. Aortic stiffness and kidney disease in an elderly population. Am J Nephrol. 2015;41:320–328.
- Mitchell GF, van Buchem MA, Sigurdsson S, et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility–Reykjavik study. Brain. 2011;134:3398–3407.
