Abstract
Purpose: The present study aimed to investigate the association between hypertension and physical/functional capacities in community-dwelling older females.
Materials and methods: Older female volunteers were dichotomized in two groups: hypertensive (n = 134) and normotensive (n = 244). Volunteers had their medical records reviewed and underwent evaluations of anthropometric data (weight, height and body mass index) and of physical and functional capacities.
Results: The results showed that hypertensive older females presented higher values for age, weight, body mass index, and resting diastolic blood pressure than normotensive older females. Normotensive older females showed a higher performance in the one-leg stand test and six-minute walk test compared with hypertensive older females. Age, body mass index, maximal walking speed, performance in the Time Up and Go and six-minute walk test, and diagnosis of diabetes mellitus type II were factors associated with hypertension using the chi-square test. However, the multivariate regression analysis indicated that performance in the six-minute walk test was the only factor associated with hypertension.
Conclusions: The patients with higher scores in the six-minute walk test, which is associated with aerobic capacity, show less odds to have clinical diagnosis of hypertension. However, hypertension was not associated with poor physical and functional capacity.
Introduction
Populations of older adults have increased exponentially in the past decades, and the United Nations (UN) and the World Health Organization (WHO) have estimated that 1.5 billion people around the world will be older than 65 years by 2050 [Citation1].
Aging is a continuous process that is characterized by alterations of physiological functions, and these alterations can lead to the development of geriatric syndromes such as sarcopenia and frailty; although there is no consensus on the method of diagnosis and on the factors associated with the progression of these syndromes, these syndromes can decrease the ability of older people to perform the activities of daily living (ADL) [Citation2–4]. Furthermore, aging is known to be positively associated with the development of chronic diseases, considering that the prevalence of these conditions increases exponentially during old age.
Hypertension (HTN) is one of the most prevalent diseases in older people, and more than 70% of the population of older women have been diagnosed with HTN [Citation5]. The main concern about this disease is its poor prognosis because patients with high blood pressure show high risk of stroke (both haemorrhagic and ischaemic) and myocardial infarction [Citation5,Citation6]. Moreover, a recent WHO report established that HTN is the main risk factor for death worldwide [Citation6].
However, although HTN is closely associated with the risk of death, it does not necessarily allow older patients to be classified as unhealthy. In fact, the Pan American Health Organization has reported that the health status in older adults is determined by the capacity to perform basic ADL (e.g., eating, walking, taking a bath) and advanced ADL (e.g., managing finances, shopping, preparing meals) [Citation7]. Furthermore, the maintenance of physical and functional capacities during old age is necessary to avoid the loss of functional independence and consequently to maintain the health status [Citation7].
In contrast to the cardiovascular and cerebrovascular perspectives on HTN, some studies have reported a possible association between HTN and low physical capacity [Citation8,Citation9]. Theoretically, high blood pressure levels can damage the arteries responsible for transporting blood to the brain, thereby limiting blood flow to the brain areas responsible for muscle contraction [Citation8,Citation10].
However, these studies have shown conflicting results, probably because of random errors (i.e. small sample size) and systematic errors (i.e. few measurements of physical capacity [gait and balance]) [Citation8,Citation9].
Therefore, the present study aimed to evaluate the relationship between HTN and physical and functional capacities in older women. We hypothesized that hypertensive older women have lower physical and functional capacity compared with normotensive women.
Materials and methods
This cross-sectional study was approved by the Research Ethics Committee of the University of Mogi das Cruzes (UMC) under protocol number 621-614 and was developed in accordance with the Declaration of Helsinki and with Resolution 196/96 of the National Health Council. Once this study was aimed to investigate the association between HTN and physical/functional capacities in community-dwelling older females, volunteers were underwent to physical and functional evaluations, anthropometric measurements, as well as evaluation of hemodynamic parameters at rest. Furthermore, medical records were reviewed determinate the prevalence of pathologies and consumption of medications. First analysis was performed based on the diagnosis of HTN. However, a further analysis was also performed according to the quartiles of SBP at rest. A representation of the study design can be observed in .
Figure 1. The phases that older women underwent before and during the study, from medical diagnosis for the evaluation of haemodynamic parameters at rest. In summary, before initiating the experimental protocol, older women have been evaluated by a medical cardiologist to confirm or not the diagnosis of HTN. Once in the study, volunteers underwent to seven physical and functional evaluations, anthropometric measurements, review of medical records and evaluation of haemodynamic parameters at rest.
Subjects
The study participants were recruited from two specialized healthcare centres for older adults in a town located in the metropolitan area of São Paulo, Brazil. Volunteers were recruited by convenience and asked verbally by the medical team and researchers about their participation in the study. All the subjects provided informed consent before enrolment.
The exclusion criteria were, use of hormone replacement and/or psychotropic drugs, cerebrovascular disease (e.g. stroke), pulmonary disease, neurological or psychiatric disease (e.g. Parkinson’s or Alzheimer’s disease), musculoskeletal disorders, comorbidities associated with greater risk of falls and any kind of dizziness, blurred vision or lightheadedness when rise or remain standing for long, which could indicate orthostatic hypertension and/or labyrinthitis. The inclusion criterion was age ≥60 years. After the application of the exclusion and inclusion criteria, 378 older women were included in the analyses.
The volunteers were subdivided into normotensive and hypertensive groups according to previous clinical diagnosis of HTN. Since both healthcare centres serve a large number of patients, and the medical team (i.e. nurse, physician and physical educator) is of limited size, the pathological conditions were simply recorded by the head physician and head nurse of each centre. A specialist (i.e. cardiologist) who was not affiliated to and was outside the centre then made the diagnosis of HTN, according to the guidelines [Citation31]. In summary, before the participants began the activities in the centres where they were recruited, a medical consultation was conducted and an extensive list of medical exams were required (e.g. fasting blood glucose, fasting blood insulin). If the patient showed any sign of HTN, such as high blood pressure levels during the first visits in the centres, she was invited to measure blood pressure levels, at least, three times, during different periods of the day at home. If her blood pressure evaluations remained elevated, she was referred to a cardiologist. After they underwent specific medical consultation (i.e. cardiologist) and performed all the specific exams (i.e. 24-h ambulatory blood pressure monitoring [ABPM], home blood pressure) [Citation31], the patients returned to the centres with a letter signed by the specialist confirming or not the diagnosis of HTN. Final diagnosis was signed by the head-physician of the centre and a nurse updated the medical records each of 6 months. The patients of the present study were recruited one week after the update of the medical records.
Physical and functional evaluations
Before the performance of the tests, an experienced researcher detailed the procedures of each test. The volunteers performed all the tests twice (except the six-minute walk test), and the higher value recorded in each test was used in the analysis. During all the tests, verbal encouragement was provided to assure that volunteers reached the best performance possible.
Handgrip strength
Maximal voluntary contraction was evaluated using a handgrip dynamometer (Jamar®, USA) while the subjects remained seated in a chair with the shoulders abducted, elbows near the trunk and flexed at 90°, and wrists in a neutral position (thumbs up). The contralateral arm remained relaxed under the thigh. To determine handgrip strength, the volunteers performed a maximal contraction during 4 s with the dominant hand [Citation11].
One-leg stand test
The one-leg stand test was performed with the volunteers standing in a unipodal stance with the dominant lower limb, the contralateral knee remaining flexed at 90°, the arms remaining crossed in front of the chest, and the head was straight. A stopwatch (Moure Jar®, China) was activated when the volunteer raised their contralateral foot off the floor and was stopped when the contralateral foot touched the floor again [Citation12].
Sit-to-stand test
Volunteers were requested to rise from a chair five times as fast as possible with their arms crossed in front of the body. The stopwatch was activated when the volunteer raised their buttocks off the chair and was stopped when the volunteer seated back.
Walking speed test
To measure walking speed, a three-meter walking speed test was performed. Volunteers were required to walk a distance of five meters at their usual and fastest possible cadences (without running). Before the evaluation, both feet of each volunteer were to remain on the starting line. Measurement was initiated when a foot reached the one-meter line and was stopped when a foot reached the four-meter line. The one-meter intervals at the beginning and end were used to avoid early acceleration and/or deceleration [Citation13].
Countermovement jump
The countermovement jump was performed to evaluate leg power. In the initial position, the volunteers stood on a jump platform (Jump System Pro, Cefise, Brazil), their feet remained approximately parallel at shoulder width, and their hands rested on their hips. When instructed, the volunteers flexed their knees at approximately 90° and jumped the maximum height possible [Citation14].
Time up and go (TUG) test
The Time Up and Go (TUG) test involved getting up from a chair without the help of the arms, walking a distance of three meters around a marker placed on the floor, coming back to the same position, and sitting back on the chair. The test began when a researcher shouted a “go!” command. The stopwatch was activated when the volunteers got up from the chair and was stopped when they were seated again [Citation15].
Six-minute walk test
The six-minute walk test was performed according to the American Thoracic Society guidelines (2002). The test was performed indoors on a 30-m track. In summary, after remaining seated for 15 min, the volunteers were asked to walk on the track as fast as possible for six minutes. In the case that the volunteers experienced chest pain, intolerable dyspnea, leg cramps, stagger, diaphoresis, pale or ashen appearance, or any other complaint, the test was interrupted. The distance walked by the volunteers in meters was used in the analysis.
Anthropometric measurements
A weight scale with a Filizola® (Brazil) stadiometer was used to measure body mass (kg) and height (cm). Body mass index (BMI) was determined by using the formula body mass (kg)/(height [cm])2.
Determination of the prevalence of pathologies and use of medications
The prevalence of pathologies and consumption of medications was determined by reviewing the medical records of each subject. As aforementioned, the head physician and the head nurse of the medical centres recorded the pathological conditions, and a specialist not affiliated with the centres made the pathologic diagnoses. These records were reviewed by two researchers (HJCJ and SSA), and data were compared.
Evaluation of hemodynamic parameters at rest
The procedures for measurement of blood pressure were adapted from the VII Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC7) [Citation16]. In summary, older women remained in a sitting position on a comfortable chair for 15 min in a quiet room. After this period, an appropriate cuff was placed at approximately the midpoint of the upper left arm (heart level). An automatic, noninvasive, and validated [Citation32] arterial blood pressure monitor (Microlife-BP 3BT0A, Microlife, Widnau, Switzerland) was used to measure systolic blood pressure (SBP), diastolic blood pressure (DBP) and heart rate (HR). During blood pressure recording, volunteers remained relaxed in the sitting position, with parallel feet at one shoulder width, both forearm and hands on the table, supinated hands, backs against the chair, without move or talk. The volunteer did not have access to blood pressure values during the measurement. The evaluation lasted approximately 80 s and was performed three times with one minute of rest among the measurements. The mean of measurements of each volunteer was used in the final analysis. Mean arterial pressure (MAP), rate pressure product (RPP), and pulse pressure (PP) were evaluated according to the following equations: MAP = [SBP + (2 * DBP)]/3, RPP = SBP * HR, and PP = SBP – DBP [Citation17]. The size of the arm cuff was selected after measuring the arm circumference of each participant (Sanny, São Paulo, Brazil). All the volunteers were evaluated within the first two months after the update of the medical records.
Statistical analysis
To determine the differences in the continuous and categorical data between the groups (i.e. normotensive and hypertensive), the Student’s t-test for independent samples and the chi-square (X2) test were performed, respectively. An a posteriori X2 test was performed to investigate the association between the dependent categorical variable (i.e. hypertensive condition) and the independent categorical variables (age, handgrip strength [n = 360], adjusted handgrip, one-leg stand test [n = 226], sit-to-stand test [n = 377], usual walking speed [n = 375], maximal walking speed [n = 376], countermovement jump [n = 358], TUG test [n = 374], six-minute walk test [n = 378], BMI, average use of drugs, diabetes mellitus type I [DMTI], diabetes mellitus type II [DMTII], arthritis, and cardiovascular disease). The median values were chosen as the cutoff values. Independent variables with a p ≤ .20 in the X2 test were included in a univariate logistic binary analysis. To be considered as an independent variable associated with HTN, the results were required to have a p ≤ .05 and a 95% confidence interval (CI 95%) that did not include the value of 1. All the analyses were conducted using the IBM SPSS Statistics, version 20.0, software (IBM Corp., Armonk, NY).
Results
The study sample included 378 older women (134 normotensive [35.5%] and 244 hypertensive [65.5%]). shows the general characteristics of the study group according to the HTN status. The hypertensive subjects were older (68.2 years vs. 66.8 years; p = .02) and presented higher values for weight (70.4 kg vs. 64.6 kg; p = .00), BMI (28.6 kg/m2 vs. 26.3 kg/m2; p = .00), and resting DBP (77.9 mmHg vs. 74.2 mmHg; p = .00) compared with the normotensive older women. The normotensive older women showed a better performance in the one-leg stand test (21.8 s vs. 18.4 s; p = .02) and in the six-minute walk test (573.6 m vs. 549 m; p = .04) compared with the hypertensive older women. No differences in the prevalence of chronic degenerative diseases were observed between the groups.
Table 1. Characteristics of the older women according to hypertension.
The associations between the categorical variables using the chi-square test (X2) are shown in . Age (0.06), BMI (0.06), maximal walking speed (0.06), TUG (0.13), six-minute walk test (0.01), and DMTII (0.08) showed significant p-values, and these variables were included in the logistic binary analysis.
Table 2. Frequency (%) of the distribution of older women according to hypertension.
presents the unadjusted OR and 95% CI results for HTN. The binary logistic regression analysis indicated that the only variable significantly associated with HTN was the six-minute walk test (unadjusted OR = 0.553; CI 95% = 1.131–2.671; p-value = .012).
Table 3. Unadjusted OR and 95% confidence intervals (CI) for hypertension.
Since blood pressure is a clinical parameter largely used to quantify cardiovascular risk and its values can represent the actual state of the patient – indicating, for example, if the pharmacological therapy is not adequate – further analyses were performed dichotomising patients according to the third quartile of SBP values at rest (144 mmHg). Thus, quartiles were divided in higher (≥75%) and lower (25%), according to SBP values.
shows the comparison among the groups regarding continuous and categorical variables in 374 patients (four were excluded due lack of data). There were no significant differences in anthropometric characteristics and physical functional tests. On the other hand, Higher quartile group showed a greater prevalence of HAS in comparison with Lower quartile group (86.0% vs. 14.0%; p < .001).
Table 4. Characteristics of older women according to blood pressure values.
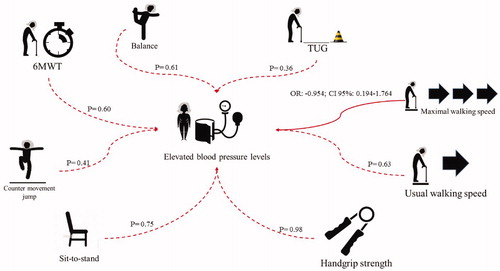
Results from Chi-square test (X2) showed p-values lower than 0.20 to age (0.11), and maximal walking speed (0.06). Both variables were added in the binary logistic regression according to p-value. Results did not confirm findings of the X2 test, once both variables were not significantly associated with high SBP values at rest (). Lastly, pathological conditions were also added in the analyses. However, variables remained not significantly associated.
Table 5. Unadjusted OR and 95% confidence intervals (CI) for SBP values.
Discussion
The main findings of the present study are: (a) high-aerobic capacity, analysed using the six-minute walk test, seems to be a protective factor against HTN; and (b) HTN and poor-controlled SBP were not significantly associated with low functionality in Brazilian community-dwelling older women. An illustration from the analyses based on the clinical diagnosis of HTN and on the quartiles of SBP levels at rest can be observed in the and .
Figure 2. Representation of chi-square test data (dotted lines) and logistic binary analyses (black lines) based on the diagnosis of hypertension.
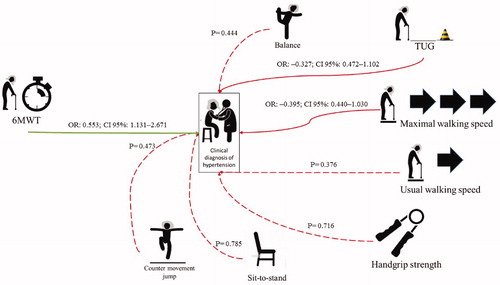
Figure 3. Representation of data from chi-square test (dotted lines) and logistic binary analysis (black lines) based on quartiles of systolic blood pressure levels at rest.
The six-minute walk test is a convenient, quick, inexpensive, safe, and well-accepted method used to evaluate functional and aerobic capacity in patients with chronic diseases, such as chronic pulmonary obstructive disease, and older patients [Citation18,Citation19]. To perform the six-minute walk test, some physiological systems (e.g. pulmonary, cardiovascular, metabolic, and neuromuscular system) must work in an integrated manner [Citation18,Citation19]. Therefore, a poor performance in the six-minute walk test can indicate a dysfunction in one or more of these systems and can lead to a decrease in the capacity to perform ADL [Citation18,Citation19].
Student’s t-test demonstrated that normotensive patients performed significantly better in the six-minute walk test than the hypertensive patients (573.6 ± 109.7 vs. 549.1 ± 115.6, respectively), and this result is corroborated by results from the binary logistic regression analysis, which indicated that better performance in the six-minute walk test was a protective factor against HTN (unadjusted OR = 0.553; CI 95% = 1.131–2.671; p-value = .012). Although some studies have proposed an association between performance in the six-minute walk test and capacity to perform ADL [Citation18,Citation19], the results of the present study do not seem to agree with this assumption.
It is important to mention that all the tests performed by normotensive and hypertensive patients in the present study were associated with mobility, capacity to perform ADL, and muscular functionality. Furthermore, the scores obtained in some of these tests (e.g. the TUG and walking speed) are used in clinical practice to diagnose syndromes associated with a decrease in muscular functionality, such as sarcopenia [Citation2,Citation3], or to evaluate the risk of falls [Citation20]. However, the binary logistic regression analysis revealed that none of the results of these tests were associated with hypertension, indicating the lack of association between HTN and poor muscular functionality in the present sample of hypertensive subjects. Therefore, the inverse association between the six-minute walk test and HTN does not seem to be related to musculoskeletal capacity.
On the other hand, an association between the six-minute walk test and aerobic capacity has been reported [Citation18], and this result seems to better explain to the negative association between the six-minute walk test and HTN. In fact, previous studies have found a strong correlation between aerobic capacity and the factors associated with HTN, such that a higher aerobic capacity has been positively associated with the plasma levels of antioxidant enzymes (e.g. glutathione peroxidase), endothelial function (e.g. flow-mediated dilation), low-fat mass, and function and structure of cardiomyocytes (e.g. stroke volume and physiological hypertrophy of the left ventricle) [Citation21,Citation22]. In turn, individuals with a lower aerobic capacity are at a higher risk of having increased levels of the protein asymmetric dimethylarginine, which inhibits the synthesis of nitric oxide, triglyceride, glucose, insulin, and markers of oxidative stress (e.g. malondialdehyde) [Citation23,Citation24]. Therefore, high aerobic capacity is probably associated with an environment that is unfavourable for the development of HTN, and this association was evident in the logistic regression analysis.
Recent reviews in the literature have described a possible association between high-blood pressure levels and mobility impairment in older people [Citation25,Citation26]. This topic has been extensively discussed in the areas of geriatrics and gerontology, and a proposed theory is that a low and controlled HTN phenotype can catalyse the development of frailty syndrome [Citation25].
However, in contrast to the hypothesis of the present study, physical and functional capacities were not associated with HTN () or poor SBP ( and ). In fact, even if the TUG and maximal walking speed tests had demonstrated an association in the X2 test, the logistic binary regression analysis indicated that this association was not significant in both analyses (diagnosis and measurement at rest).
Cross-sectional studies have found an association between HTN and physical function (primarily gait and balance); however, the results are conflicting. Hausdorff et al. [Citation9] observed worse performance in balance and gait tests in hypertensive patients compared with normotensive patients. On the other hand, recent results from Acar et al. [Citation8] did not find differences in the performance of a battery of balance tests (i.e. clinical tests of sensory interaction and balance) among hypertensive and normotensive patients.
One of the most important differences between the present study and the aforementioned studies is the sensitivity and specificity of the tools. Similar to the procedure used by Hausdorff et al. (2003) [Citation9], in the present study, balance was examined on a stable platform, which resulted in a limited capacity to infer data. However, Acar et al. [Citation8] evaluated balance using four conditions, two of which included unstable surfaces. Another important consideration about the experiments is the age of the study sample. Our study sample was approximately 10 years younger than the samples of the aforementioned studies. This seems to be an important issue to consider because longitudinal multicentre studies have demonstrated that the association between HTN and impairment of functional mobility is common in older adults or at least adults more than 75 years [Citation10]. Therefore, it is possible that this phenomenon is time dependent [Citation25]. However, the logistic binary analysis did not find any significant association between age and HTN.
To the best of our knowledge, the present study is the first to analyse a possible association between HTN and muscle power (i.e. via the countermovement jump test). Previous evidence has demonstrated that muscle power is more strongly associated with muscle functionality than with other physical capacities (i.e. muscle strength) [Citation27]. Furthermore, muscle power has been associated with balance [Citation28].
Continuous vascular damage in the arteries responsible for the transport of blood to the brain areas responsible for mobility (e.g. motor cortex) is one of the mechanisms proposed to explain the decrease of muscle functionality caused by HTN [Citation8,Citation10]. Therefore, it is possible to propose that physical capacities (e.g. muscle power and muscle strength) at the onset of the vascular damage caused by HTN could be associated with hypertension, although these capacities are not, necessarily, associated with muscle functionality. However, these results were not observed in the present study.
Mainly two systematic errors clearly limit the ability to infer conclusions from the data in the present study. Sarcopenia is a multifactorial syndrome with a high prevalence among aging adults [Citation2,Citation3]. This syndrome is primarily characterized by a decrease in muscle mass accompanied by dynapenia (i.e. low-muscle strength) and/or low functionality [Citation2,Citation3]. The main concern about sarcopenia is its association with poor prognosis, which can lead to an increased risk of disability and frailty in older adults [Citation2,Citation3]. Evidence in the literature has demonstrated an association between sarcopenia and indicators of arterial stiffness, which is one of the factors related to the increase in the post-cardiac load, and consequently blood pressure [Citation17,Citation29]. Furthermore, a recent study with a cohort of approximately 5000 older Korean adults showed that sarcopenic patients have higher odds of hypertension compared with non-sarcopenic patients [Citation30].
In the present study, muscle mass was not evaluated and this is a limitation of the present study, once an increased number evidences have been indicating a “sarcopenic hypertensive phenotype”. Muscle mass evaluation could collaborate to understand if sarcopenia is confounding in the relationship between HTN and functionality. Another limitation of the present study is the lack of information about the time of diagnosis of HTN. As previously mentioned, Muller et al. [Citation25] suggested that the association between HTN and impaired mobility is time-dependent and that the time of diagnosis can be considered an independent variable.
However, even if such evaluations were not carried out, the present study shows some strengths, such as: large sample size, several physical and functional evaluations, as well as the analyses of older women from different ages (i.e. <75 and ≥75). Regarding physical and functional evaluations, increased the number of analyses seems to be crucial and collaborate to amplify previous published data, since other studies limited the physical evaluation to balance. The study of other physical functions, such as aerobic capacity, demonstrated to be important, since data of the present study indicate that the maintenance of aerobic capacity can be a protective factor against HTN in older women. In turn, further analyses with very older women (≥75), allow us to do not support the hypothesis that physical and functional impairment are time-dependent.
Therefore, future experiments must include the evaluation of muscle mass and other factors associated with HTN, such as the time of diagnosis, in the experimental design, once the knowledge about relation of both the factors with hypertension and functionality will collaborate to elucidate this phenomenon.
In conclusion, higher scores in the six-minute walk test, which is associated with aerobic capacity, acted as a protective factor against hypertension. However, hypertension was not associated with poor physical and functional capacity.
Acknowledgments
The authors are grateful to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for funding this research via scholarships to HJCJ and RYA. BR had financial support from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and CNPq (BPQ). The authors are also grateful to Daisy dos Reis and Flávio Romano of the facility for older adults, and all researchers of the Research Group on Chronic-Degenerative Diseases of Mogi das Cruzes University (Grupo de Pesquisa em Doenças Crônico-Degenerativas da Universidade de Mogi das Cruzes–GEDCD/UMC) for their support.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Desa U. World population prospects, the 2012 revision. New York: Department for Economic and Social Affairs; 2013.
- Chen L-K, Liu L-K, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15:95–101.
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423.
- Sewo Sampaio PY, Sampaio RAC, Coelho Júnior HJ, et al. Differences in lifestyle, physical performance and quality of life between frail and robust Brazilian community- dwelling elderly women. Geriatr Gerontol Int. 2016;16:829–835.
- Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics-2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245.
- World Health Organization. Global health risks: mortality and burden of disease attributable to selected major risks. Geneva: World Health Organization; 2009.
- Moraes EN. Atenção à saúde do Idoso: Aspectos Conceituais. Brazil: Organização Pan-Americana da Saúde; 2012.
- Acar S, Demırbüken İ, Algun C, et al. Is hypertension a risk factor for poor balance control in elderly adults? J Phys Ther Sci. 2015;27:901.
- Hausdorff JM, Herman T, Baltadjieva R, et al. Balance and gait in older adults with systemic hypertension. Am J Cardiol. 2003;91:643–645.
- Rosano C, Longstreth WT, Boudreau R, et al. High blood pressure accelerates gait slowing in well-functioning older adults over 18-years of follow-up. J Am Geriatr Soc. 2011;59:390–397.
- Roberts HC, Denison HJ, Martin HJ, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40:423–429.
- Briggs RC, Gossman MR, Birch R, et al. Balance performance among noninstitutionalized elderly women. Phys Ther. 1989;69:748–756.
- Yamada M, Nishiguchi S, Fukutani N, et al. Prevalence of sarcopenia in community-dwelling Japanese older adults. J Am Med Dir Assoc. 2013;14:911–915.
- Ramírez-Campillo R, Castillo A, Carlos I, et al. High-speed resistance training is more effective than low-speed resistance training to increase functional capacity and muscle performance in older women. Exp Gerontol. 2014;58:51–57.
- Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148.
- Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572.
- Coelho Júnior HJ, Aguiar SDS, Gonçalves IDO, et al. Sarcopenia is associated with high pulse pressure in older women. J Aging Res. 2015;2015:109824.
- Ross RM, Murthy JN, Wollak ID, et al. The six minute walk test accurately estimates mean peak oxygen uptake. BMC Pulm Med. 2010;10:31
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111.
- Kenny R, Rubenstein LZ, Tinetti ME, et al. Summary of the updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc. 2011;59:148–157.
- Buscemi S, Canino B, Batsis JA, et al. Relationships between maximal oxygen uptake and endothelial function in healthy male adults: a preliminary study. Acta Diabetol. 2013;50:135–141.
- Pialoux V, Brown AD, Leigh R, et al. Effect of cardiorespiratory fitness on vascular regulation and oxidative stress in postmenopausal women. Hypertension. 2009;54:1014–1020.
- Tanahashi K, Akazawa N, Miyaki A, et al. Plasma ADMA concentrations associate with aerobic fitness in postmenopausal women. Life Sci.2014;108:30–33.
- Wisløff U, Najjar SM, Ellingsen Ø, et al. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science. 2005;307:418–420.
- Muller M, Smulders YM, de Leeuw PW, et al. Treatment of hypertension in the oldest old: a critical role for frailty? Hypertension. 2014;63:433–441.
- Sorond FA, Cruz-Almeida Y, Clark DJ, et al. Aging, the central nervous system, and mobility in older adults: neural mechanisms of mobility impairment. J Gerontol a Biol Sci Med Sci. 2015;70:1526–1532.
- Bean JF, Leveille SG, Kiely DK, et al. A comparison of leg power and leg strength within the InCHIANTI study: which influences mobility more? J Gerontol a Biol Sci Med Sci. 2003;58:728–733.
- Orr R, Raymond J, Singh MF. Efficacy of progressive resistance training on balance performance in older adults : a systematic review of randomized controlled trials. Sports Med. 2008;38:317–343.
- Sampaio RA, Sewo Sampaio PY, Yamada M, et al. Arterial stiffness is associated with low skeletal muscle mass in Japanese community-dwelling older adults. Geriatr Gerontol Int. 2014;14:109–114.
- Han K, Park YM, Kwon HS, et al. Sarcopenia as a determinant of blood pressure in older Koreans: findings from the Korea National Health and Nutrition Examination Surveys (KNHANES) 2008–2010. PLoS One. 2014;9:e86902.
- Sociedade, Brasileira de Hipertensão, Sociedade Brasileira de Cardiologia, and Sociedade Brasileira de Nefrologia. VI Brazilian guidelines on hypertension. Arq Bras Cardiol. 2010;95(1 Suppl):1–51.
- Cuckson AC, Reinders A, Shabeeh H, et al. British Hypertension Society. Validation of the Microlife BP 3BTO-A oscillometric blood pressure monitoring device according to a modified British Hypertension Society protocol. Blood Press Monit. 2002;7:319–324.
