Abstract
Purpose: The four SMILE studies demonstrated that early administration of zofenopril following acute myocardial infarction is prognostically beneficial compared to placebo or other angiotensin-converting enzyme (ACE) inhibitors. In the present retrospective pooled analysis of individual SMILE studies, we evaluated the efficacy of zofenopril on cardiovascular (CV) outcomes in 1880 hypertensive and 1662 normotensive patients.
Materials and methods: Four hundred and forty-nine hypertensives and 486 normotensives were treated with placebo, 980 and 786 with zofenopril 30–60 mg daily, 252 and 259 with lisinopril 5–10 mg daily, 199 and 131 with ramipril 10 mg daily, for 6 to 48 weeks.
Results: The 1-year risk of death or hospitalization for CV causes was significantly reduced with zofenopril and lisinopril vs. placebo in both hypertensive (HR: 0.65; 95%CI: 0.48–0.86; p = .003 and .60, .36–.99; p = .049, respectively) and normotensive patients (0.56, 0.42–0.75; p = .0001 and .51, .28–.90; p = .020), whereas this was not the case for ramipril (hypertensives: 1.02, 0.69–1.52; p = .918; normotensives: 0.91, 0.59–1.41; p = .670). Zofenopril significantly reduced the risk of CV outcomes vs. the other two ACE-inhibitors pooled together in hypertensive (0.76; 0.58–0.99; p = .045), but not in normotensive patients (0.77; 0.55–1.10; p = .150).
Conclusions: In cardiac patients of the SMILE studies with arterial hypertension treatment with the ACE-inhibitor zofenopril was beneficial in reducing the 1-year risk of CV events as compared to placebo and ramipril. An efficacy similar to that of zofenopril was observed with lisinopril.
Introduction
A positive history for arterial hypertension is frequently reported in patients with acute myocardial infarction (AMI) and such condition is associated with an increased risk of cardiovascular morbidity and mortality during both early and long-term course of AMI [Citation1]. The increased incidence of cardiovascular conditions after AMI in hypertensive patients seems to be linked to endothelial damage, atherosclerosis, insulin resistance, left ventricular hypertrophy and ventricular arrhythmias [Citation2,Citation3]. A large evidence supports the rationale for a cardioprotective role of angiotensin-converting enzyme (ACE) inhibitors both in vitro and in clinical studies. It has been reported that ACE-inhibitors favour vasodilatation vs. vasoconstriction, limit neurohormonal activation during the ischaemia, reduce oxidative stress, thus improving endothelial function and delaying atherosclerosis development, inhibit platelet activation and reverse negative vascular remodelling [Citation4]. In particular, in post-AMI patients with antecedent hypertension potential clinical benefits of ACE-inhibitors are related to prevention or attenuation of adverse ventricular remodelling and infarct expansion, more frequently observed in these patients [Citation5]. This observation may explain why treatment with ACE-inhibitors yields a greater benefit on mortality risk in hypertensive than in normotensive patients. In the TRAndolapril Cardiac Evaluation (TRACE) study, following AMI and in the presence of left ventricular dysfunction (LVD), trandolapril reduced the mortality risk by 41% in hypertensive patients and by 15% in normotensive patients [Citation6]. In the Acute Infarction Ramipril Efficacy (AIRE) study, ramipril reduced the all-cause mortality risk by 37% in hypertensive patients and by 22% in normotensive patients in the presence of heart failure after AMI [Citation7].
Zofenopril is a sulfhydryl-containing, lipophilic, ACE inhibitor, with ancillary cardioprotective properties specifically recommended to treat myocardial infarction and heart failure [Citation8,Citation9]. Its unique mechanism of action combines ACE-inhibition within plasma and tissue, and anti-oxidant properties, thus effectively controlling blood pressure, left ventricular function, and myocardial ischemia burden [Citation10,Citation11]. The double-blind, randomized, parallel group, prospective trials of the SMILE Project, which involved more than 3,600 patients with coronary heart disease, demonstrated that early AMI treatment with zofenopril may reduce mortality and morbidity, also when combined with acetylsalicylic acid, with a greater extent than placebo, lisinopril and ramipril [Citation12–15]. Based on this evidence, zofenopril is currently indicated for early treatment of patients with AMI, with or without heart failure symptoms, who are haemodynamically stable and have not received thrombolytic therapy [Citation10].
Two previous retrospective analyses of the SMILE-1 and SMILE-4 study confirmed the superiority of zofenopril vs. placebo or vs. ramipril in the prevention of cardiovascular outcomes also in the subgroup of high-risk cardiac patients with arterial hypertension [Citation16,Citation17]. A previous pooled analysis of the four SMILE studies, focused on the effect of zofenopril vs. placebo and vs. the other ACE-inhibitors pooled together (lisinopril and ramipril) showed a good overall efficacy of the drug and introduced the evidence that such efficacy is well maintained regardless of age, gender, diabetes, hypertension or presence of cardiovascular risk factors [Citation18]. However, no specific post hoc analyses in hypertensive patients were done for the SMILE-2 study, assessing the efficacy of zofenopril vs. lisinopril [Citation13], for the SMILE-3 study, which evaluated the efficacy of zofenopril vs. placebo on ischaemic burden [Citation14], and for the pooled datasets, separately for placebo, zofenopril, lisinopril and ramipril [Citation18]. To bridge this gap, we decided to perform a retrospective pooled individual data analysis considering all the SMILE studies, aiming at providing more robust, consistent and powerful results on the efficacy of zofenopril vs. placebo and the active comparators (lisinopril and ramipril) on the prevention of cardiovascular morbidity and mortality.
Methods
Study design and population
The four double-blind, randomized, parallel-group SMILE studies, compared the efficacy and safety of zofenopril with that of placebo (SMILE-1 and 3) [Citation12,Citation14], lisinopril (SMILE-2) [Citation13] or ramipril (SMILE-4) [Citation15] in European men and non-pregnant women with AMI. Inclusion criteria were as follows: (i) AMI diagnosis within less than 24 h, not eligible for thrombolytic therapy for late hospital admission or contraindication to systemic fibrinolysis (SMILE-1) [Citation12], (ii) a confirmed diagnosis of AMI and a prior thrombolytic treatment within 12 h from onset of AMI clinical symptoms (SMILE-2) [Citation13]; (iii) a recent AMI (within 6 ± 1 weeks) with preserved left ventricular ejection fraction (LVEF > 40%), treated with thrombolytic therapy and ACE-inhibitors (SMILE-3) [Citation14] and (iv) early myocardial infarction (<24 h), treated or not with thrombolysis, with primary percutaneous transluminal angioplasty or coronary artery by-pass graft, and with clinical and/or echocardiographic evidence of LVD (SMILE-4) [Citation15]. All studies were conducted according to the Guidelines for Good Clinical Practice and the Declaration of Helsinki and were approved by the Ethics Committee of each participating centre. Written informed consent was obtained from each patient before enrolment.
Treatments
Eligible patients were double-blind randomly allocated to treatment with zofenopril or comparator (placebo, lisinopril or ramipril), in addition to standard recommended therapy for AMI. Treatments and randomization procedures were described in the original studies. Briefly, the first zofenopril dosing was 7.5 mg twice daily on day 1 and 2, followed by 15 mg twice daily on day 3 and 4, and 30 mg twice daily from day 5 onwards. Up-titration was allowed if systolic blood pressure remained >100 mmHg and if there were no signs or symptoms of hypotension. Similarly, up-titration scheme dosing was applied to lisinopril (up to 10 mg once-daily) and ramipril (up to 5 mg twice-daily). For all studies, duration of treatment and follow-up periods overlapped, except for the SMILE-1 Study. In this trial, the patients stopped the double-blind treatment with the study medication after 6 weeks, and continued AMI therapy with their other medications for additional 48 weeks.
Statistical analysis
Since all the four SMILE Studies provided information on fatal and non-fatal CV events, the primary study endpoint of this retrospective analysis was set to the 1-year combined occurrence of death or hospitalization for CV causes (AMI, severe congestive heart failure, angina, revascularization procedure or significant decline in LVEF). The efficacy end-point was calculated after weighing for the number of subjects contributing from each study. The analysis was carried out on the intention-to-treat population, made up of all randomized patients treated with at least one dose of study medication and documenting at least once the measure of the primary efficacy assessment, even in case of protocol violation or premature withdrawal from the study. The analysis was based on the between-treatment comparison of the primary study endpoint, separately assessed in hypertensive and normotensive patients. Arterial hypertension was considered to be present when patients had previously been informed of a diagnosis of such a condition for which specific pharmacological therapy was still being taken.
The baseline characteristics and the distribution of variables in the study populations and subgroups were compared using a Chi-square test for categorical variables and a Student t test for continuous variables. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated by a Cox proportional-hazard regression model. In order to account for the different duration of follow up among the four studies, the relative risk of CV morbidity and mortality was assessed using a time-dependent Cox regression model and corresponding survival curves were drawn. Results were adjusted for confounding factors such as country, gender (male vs. female), age (<65 vs. ≥65 years), body mass index (BMI < 30 vs. ≥30 kg/m2), cardiovascular risk factors (yes vs. no).
All p values are two-sided and the minimum level of statistical significance was set at p < .05. Data are shown as mean ± SD or as mean and 95% confidence interval or as absolute (n) and relative (%) frequencies.
Results
Patient characteristics
Among 3630 available patients, 1556 (43%) were enroled in the SMILE-1, 1024 in the SMILE-2 (28%), 334 in the SMILE-3 (9%) and 716 in the SMILE-4 Study (20%). Data from 88 patients were not analyzed because of lack of information on hypertensive status. Therefore, 3542 patients were included in the analysis: 1880 patients (53.1%) were hypertensive and 1662 (46.9%) normotensive. Among the hypertensive patients, 449 were treated with placebo (23.9%), 980 with zofenopril (52.1%) and 451 with lisinopril or ramipril (24.0%). In the normotensive subgroup, 486 patients received placebo (29.3%), 786 zofenopril (47.3%) and 390 lisinopril or ramipril (23.4%).
As shown in baseline characteristics were heterogeneous across the four treatment groups.
Table 1. Demographic and clinical characteristics of the study population summarized by hypertension vs. normotension status and type of treatment.
Cardiovascular outcomes in hypertensive vs. normotensive patients
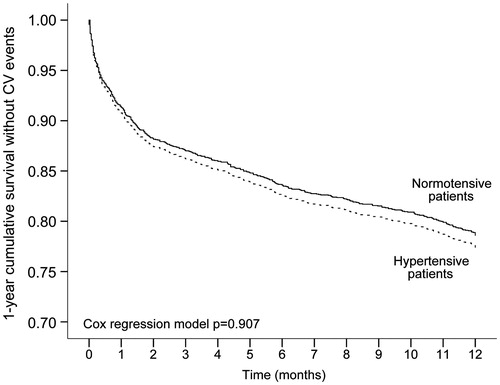
Three hundred seventy-nine hypertensive patients (20.2%) and 284 normotensive patients (17.1%) reported a major CV event during the 1-year follow up, without any significant difference between the two groups (HR:1.01; 95%CI: 0.86–1.19; p = .907). As shown in , the survival rate similarly decreased in both hypertensive and normotensive patients. Survival time averaged to 9.9 months (95%CI: 9.7–10.1 months) in patients with hypertension and to 10.1 months (95%CI 9.9–10.3) in those without hypertension (p = .115 log rank test, between groups).
Cardiovascular outcomes according to the type of treatment in hypertensive patients
One hundred sixty-nine hypertensive patients (17.2%) under zofenopril treatment, 32 (12.7%) under lisinopril, 79 (39.7%) under ramipril and 99 (22.0%) under placebo had a CV event. The risk of CV events was significantly reduced with zofenopril vs. placebo (HR: 0.65; 95%CI: 0.48–0.86; p = .003) and with lisinopril vs. placebo (HR: 0.60; 95%CI: 0.36–0.99; p = .049), whereas no significant effect was observed for ramipril vs. placebo (HR: 1.02; 95%CI: 0.69–1.52; p = .918). When pooled together, lisinopril and ramipril did not significantly reduce CV morbidity and mortality compared to placebo: 111 patients (24.6%) reported CV events under other ACE-inhibitors (32 treated with lisinopril and 79 with ramipril) and the HR was 0.83 (95%CI: 0.57, 1.21; p = .338). Comparing zofenopril with the other ACE-inhibitors, the risk of CV events was significantly reduced by 24% (HR: 0.76; 95%CI: 0.58–0.99; p = .045).
As shown in (left panel), 1-year survival rate without any major CV event was significantly higher under active treatments than under placebo. However, lisinopril and zofenopril were more effective than ramipril (, left panel).
Figure 2. Cumulative survival without events during 1-year of follow-up in hypertensive patients treated with placebo (n = 449), zofenopril (n = 980) or other ACE-inhibitors (n = 451), and in normotensive patients (n = 486 placebo, n = 786 zofenopril, n = 390 other ACE-inhibitors). p Values are from the Cox regression analysis.
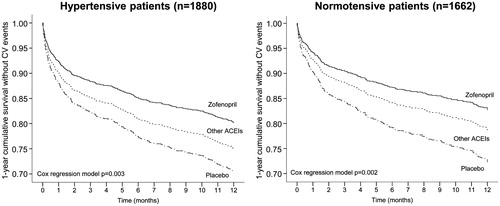
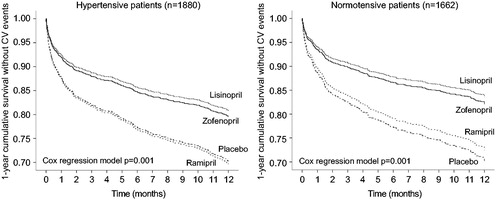
Figure 3. Cumulative survival without events during 1-year of follow-up in hypertensive patients treated with placebo (n = 449), zofenopril (n = 980), lisinopril (n = 252) or ramipril (n = 199), and in normotensive patients (n = 486 placebo, n = 786 zofenopril, n = 259 lisinopril, n = 131 ramipril). p Values are from the Cox regression analysis.
Cardiovascular outcomes according to the type of treatment in normotensive patients
In the normotensive subgroup, 108 patients (13.7%) treated with zofenopril, 23 (8.9%) treated with lisinopril, 43 (32.8%) treated with ramipril and 110 patients (22.6%) under placebo reported a CV event during the 1-year of follow-up. The risk of mortality and morbidity was significantly reduced vs. placebo under treatment with zofenopril (HR: 0.56; 95%CI: 0.42–0.75; p = .0001) and lisinopril (HR: 0.51; 95%CI: 0.28–0.90; p = .020), but not with ramipril (HR: 0.91; 0.59–1.41; p = .670). The treatment with other ACE-inhibitors had a reduced risk of CV events in comparison to placebo (16.9% reported an event; HR: 0.76; 95%CI: 0.50–1.15), but the difference was not statistically significant (p = .194). Zofenopril reduced the risk of major CV outcomes compared to lisinopril and ramipril, but the between-treatment difference was not statistically significant (HR: 0.77; 95%CI: 0.55–1.10; p = .150).
Cumulative survival rates were higher under zofenopril and other ACE-inhibitors than under placebo (, right panel), with larger effects under zofenopril and lisinopril than under ramipril (, right panel).
Cardiovascular outcomes in hypertensive vs. normotensive patients according to the type of treatment
A logistic regression analysis, which accounted for the presence or absence of hypertension, documented a 1-year risk of CV events significantly lower under zofenopril vs. placebo (HR: 0.61; 95%CI: 0.49–0.74; p = .0001) and lisinopril vs. placebo (HR: 0.56; 95%CI: 0.39–0.82; p = .003), but not with ramipril (HR: 0.96; 95%CI: 0.71–1.28; p = .768). When pooled together, lisinopril and ramipril showed a better effect than placebo [HR: 0.78; 95%CI: 0.60–1.02], but the difference did not achieve the statistical significance (p = .078). Zofenopril was slightly more effective in terms of prevention of CV morbidity and mortality than the other ACE-inhibitors [HR: 0.79; 95%CI: 0.64–0.97; p = .024].
Blood pressure at study end
As shown in , minor differences in blood pressure values were observed among the treatment groups at study end. In the hypertensive group, systolic blood pressure did not significantly differ across treatments, whereas diastolic blood pressure was significantly lower under lisinopril or ramipril than under zofenopril or placebo. This was the case for heart rate too. In the normotensive group systolic blood pressure was significantly higher in lisinopril or ramipril-treated patients. Diastolic blood pressure and heart values did not differ among treatments in normotensives.
Table 2. Blood pressure and heart rate at study end by hypertension vs. normotension status and type of treatment.
Discussion
This retrospective pooled individual data analysis of the four SMILE studies investigated the efficacy of zofenopril on cardiovascular morbidity (AMI, severe congestive heart failure, angina, revascularization procedure or significant decline in LVEF) and mortality specifically in hypertensive patients. We recorded similar rates of major CV events in hypertensive and normotensive patients. In both groups, an active treatment with ACE-inhibitors tended to improve the 1-year survival rate without CV events compared to placebo, but a significant reduction was observed only after zofenopril treatment (hypertensive – 35% and normotensive – 44%) and lisinopril treatment (hypertensive – 40% and normotensive – 49%). The direct comparison between the active treatments in the hypertensive subgroup, indicated a significantly larger effect of zofenopril and lisinopril as compared to ramipril. Interestingly, the magnitude of the reduction on CV outcome occurrence related to ACE-inhibitors was slightly higher in normotensive than in hypertensive patients. These results add to and strengthen those of previous post hoc analyses performed on the SMILE-1 and 4 studies [Citation12,Citation15], by including data from the SMILE-2 and SMILE-3 studies. We also used a robust analytic approach based on pooling study data at an individual level, adjusting for heterogeneity of the different study populations and designs, and accounting for the different length of the observation in the four studies.
A previous post hoc analysis on patients enrolled in the SMILE-1 study demonstrated that after zofenopril treatment the 1-year risk of death was reduced by 39% in hypertensive patients and by 23% in normotensive patients compared to placebo, and the reduction in the 6-week incidence of death or severe congestive heart failure was 40% in the hypertensive and 11% in the normotensive population [Citation16]. One limitation of the SMILE-1 post hoc analysis was the lack of information about the prevalence of left ventricular hypertrophy in the population [Citation16]. In the SMILE-4 population, which included patients with LVEF < 45% after AMI, [Citation17] zofenopril resulted more effective than ramipril in reducing the relative risk of major cardiovascular events and its effect was more evident in hypertensive than in normotensive patients [Citation17]. LVD is linearly related to systemic blood pressure; early administration of ACE-inhibitors may prevent LVD progression and infarct expansion that are more pronounced in patients with prior hypertension [Citation6,Citation7,Citation19,Citation20]. In a non-randomized, prospective study, after six months from AMI, hypertensive patients showed a significant increase in end diastolic volume index and in end systolic volume index, while normotensive ones ameliorated the ejection fraction, thus suggesting that antecedent hypertension affects ventricular cavity dilatation after AMI [Citation21]. Therefore, preserving left ventricular function should be an important clinical outcome for pharmacological post-AMI treatment. In our post hoc pooled analysis of the SMILE studies, we considered patients with both preserved LVEF and LVD, because the inclusion criteria were the preserved left ventricular function for the SMILE-3 study [Citation14] and a LVEF <45% for the SMILE-4 study [Citation15]. The positive effect of ACE-inhibitors in patients with LVD is well documented [Citation22–24]. However, blocking the renin angiotensin system with both angiotensin receptor blockers or ACE-inhibitors has demonstrated to have beneficial effects also in patients with preserved left ventricular systolic function [Citation25]. Therefore, although current guidelines do not recommend the long-term use of ACE-inhibitors in normotensive patients without LVD [Citation26], zofenopril treatment in the early phase after AMI may be beneficial also for this class of patients, as the results of our pooled individual data analysis suggest.
Zofenopril has antioxidant properties at clinically achievable tissue concentrations that further explain its cardioprotective role. In endothelial cells, zofenopril enhances nitric oxide production, attenuates atherosclerotic lesion development, and inhibits cellular adhesion molecule expression by reducing oxygen reactive species. These peculiar characteristics may be advantageous to control the cardiac hypertrophy, independently of blood-pressure reducing effects [Citation9]. The results of the SMILE-3 (ISCHEMIA) study supported the cardioprotective role of zofenopril in patients with normal left ventricular function and demonstrated a lower rate of development and progression of congestive heart failure with the ACE-inhibitor treatment [Citation14]. Other studies in hypertensive patients, with or without metabolic abnormalities, showed additional tissue protective effects of zofenopril, with the reduction of progression of organ damage, related to reduction in blood pressure and oxidative stress, and improvement in the NO pathway [Citation27–29].
Compared to placebo, zofenopril seems to provide similar clinical outcomes to lisinopril and better than ramipril, thus suggesting that the efficacy among ACE-inhibitors may not be the same. These results partially differ from those of a non-randomized, observational study in which no differences were observed in the risk of mortality and reinfarction among patients treated with trandolapril, ramipril, enalapril, captopril, perindopril and other ACE-inhibitors, suggesting a class effect rather than a specific activity of the single ACE-inhibitor [Citation30]. Although studies in animal and humans (including hypertensive patients) have shown that zofenopril may have specific cardioprotective and vascular effects, which might be related either to interference with bradykinin metabolism or to preservation of protein sulfhydryl groups, [Citation31–33] we cannot exclude that the superiority of zofenopril vs. the other ACE-inhibitors with respect to placebo might be simply related to a larger number of subjects included in this group and to some heterogeneity across the studies. We also cannot exclude that the larger benefit achieved with zofenopril may be related to a larger effect on blood pressure, although blood pressure values in hypertensive patients were higher at baseline and at study end under zofenopril than under the other ACE-inhibitors, and thus such a possibility is unlikely.
The study has some limitations. The post hoc nature of this analysis avoided to control some relevant differences in the inclusion criteria, treatment duration, and primary outcomes among the four SMILE studies. In the between-treatments analysis, the heterogeneity in baseline characteristics might have tended to decrease the sensitivity to show interaction. However, such differences are inherent to all pooled analysis and to reduce the bias introduced into ascertainment of the average effects, we adjusted comparisons for confounding variables and we used individual patients’ data instead of averages. We also ran a specific analysis which took into account the different length of the observation in the four SMILE studies, though we acknowledge that different durations of the follow-ups could have interfered with the appropriateness of the results. Another limitation is that the distribution of thrombolyzed and non-thrombolyzed patients, and patients with or without LVD was not homogeneous among groups. These aspects are directly related to antecedent history of hypertension, therefore they should be accounted when the risk of CV events is investigated in the hypertensive patients. The first SMILE study was carried out in the early nineties, whereas the last SMILE study 10–15 years later: the old fashion design and treatment used in the first SMILE study might not make the translation of the results into the clinical practice very actual or realistic. We did not observe differences in the overall benefit of treatments between hypertensive and normotensive patients. However, this might be expected given the fact that all patients were at high CV risk due to the concurrent AMI, regardless of their hypertensive status, and all of them were treated with CV drugs, including ACE-inhibitors, as recommended by guidelines.
Finally, we anticipated some of the results presented in this paper in the main publication of the pooled dataset [Citation18]. However, at variance from the previous publication, in the present work we also showed data separately for the three ACE-inhibitors (zofenopril, lisinopril and ramipril). We acknowledge that further studies should be designed to prospectively investigate the effect of zofenopril in hypertensive and normotensive patients after AMI.
In conclusion, this post hoc study confirms the favourable effects of treatment with ACE-inhibitors early after AMI in high-risk cardiac patients with arterial hypertension, with different features according to the type of molecule. Treatment with the ACE-inhibitor zofenopril was more beneficial in reducing the 1-year risk of CV events as compared to placebo and ramipril. An efficacy similar to that of zofenopril was observed with lisinopril. Our results suggest that early treatment of AMI patients with ACE-inhibitors could represent a good pharmacological strategy both for patients with antecedent hypertension and normotensive ones.
Disclosure statement
Conflict of interest of Prof Claudio Borghi: consultancy for Boheringer Ingelheim, Menarini International, Sanofi, Amgen, Takeda, Novartis, Ely Lilly and Servier. None of the other authors has any conflict of interest to disclose.
Additional information
Funding
References
- Pedrinelli R, Ballo P, Fiorentini C, et al. Gruppo di Studio Ipertensione e Cuore, Societa’ Italiana di Cardiologia. Hypertension and acute myocardial infarction: an overview. J Cardiovasc Med (Hagerstown). 2012;13:194–202.
- Fadl YY, Zareba W, Moss AJ, et al. History of hypertension and enhanced thrombogenic activity in postinfarction patients. Hypertension. 2003;41:943–949.
- Richards AM, Nicholls MG, Troughton RW, et al. Antecedent hypertension and heart failure after myocardial infarction. J Am Coll Cardiol. 2002;39:1182–1188.
- Bertrand ME. Provision of cardiovascular protection by ACE inhibitors: a review of recent trials. Curr Med Res Opin. 2004;20:1559–1569.
- Picariello C, Lazzeri C, Attanà P, et al. The impact of hypertension on patients with acute coronary syndromes. Int J Hypertens. 2011;2011:563657.
- Gustafsson F, Torp-Pedersen C, Køber L, et al. Effect of angiotensin converting enzyme inhibition after acute myocardial infarction in patients with arterial hypertension. TRACE Study Group, Trandolapril Cardiac Event. J Hypertens. 1997;15:793–798.
- Spargias K, Ball S, Hall A. The prognostic significance of a history of systemic hypertension in patients randomised to either placebo or ramipril following acute myocardial infarction: evidence from the AIRE study. Acute Infarction Ramipril Efficacy. J Hum Hypertens. 1999;13:511–516.
- Ambrosioni E. Defining the role of zofenopril in the management of hypertension and ischemic heart disorders. Am J Cardiovasc Drugs. 2007;7:17–24.
- Evangelista S, Manzini S. Antioxidant and cardioprotective properties of the sulphydryl angiotensin-converting enzyme inhibitor zofenopril. J Int Med Res. 2005;33:42–54.
- Borghi C, Bacchelli S, Degli Esposti D. Long-term clinical experience with zofenopril. Expert Rev Cardiovasc Ther. 2012;10:973–982.
- Borghi C, Bacchelli S, Degli Esposti D, et al. A review of the angiotensin-converting enzyme inhibitor, zofenopril, in the treatment of cardiovascular diseases. Expert Opin Pharmacother. 2004;5:1965–1977.
- Ambrosioni E, Borghi C, Magnani B. The effect of the angiotensin-converting-enzyme inhibitor zofenopril on mortality and morbidity after anterior myocardial infarction. The Survival of Myocardial Infarction Long-Term Evaluation (SMILE) Study Investigators. N Engl J Med. 1995;332:80–85.
- Borghi C, Ambrosioni E. Survival of Myocardial Infarction Long-term Evaluation-2 Working Party. Double-blind comparison between zofenopril and lisinopril in patients with acute myocardial infarction: results of the Survival of Myocardial Infarction Long-term Evaluation-2 (SMILE-2) study. Am Heart J. 2003;145:80–87.
- Borghi C, Ambrosioni E. Survival of Myocardial Infarction Long-term Evaluation Study Group. Effects of zofenopril on myocardial ischemia in post-myocardial infarction patients with preserved left ventricular function: the Survival of Myocardial Infarction Long-term Evaluation (SMILE)-ISCHEMIA study. Am Heart J. 2007;153:445.e7–14.
- Borghi C, Ambrosioni E, Novo S, SMILE-4 Working Party, et al. Comparison between zofenopril and ramipril in combination with acetylsalicylic acid in patients with left ventricular systolic dysfunction after acute myocardial infarction: results of a randomized, double-blind, parallel-group, multicenter, European study (SMILE-4). Clin Cardiol. 2012;35:416–423.
- Borghi C, Bacchelli S, Esposti DD, et al. Effects of the administration of an angiotensin-converting enzyme inhibitor during the acute phase of myocardial infarction in patients with arterial hypertension. SMILE Study Investigators. Survival of Myocardial Infarction Long-term Evaluation. Am J Hypertens. 1999;12:665–672.
- Borghi C, Ambrosioni E, Omboni S, SMILE-4 Working Party, et al. Zofenopril and ramipril and acetylsalicylic acid in postmyocardial infarction patients with left ventricular systolic dysfunction: a retrospective analysis in hypertensive patients of the SMILE-4 study. J Hypertens. 2013;31:1256–1264.
- Borghi C, Omboni S, Reggiardo G, et al. Cardioprotective role of zofenopril in patients with acute myocardial infarction: a pooled individual data analysis of four randomised, double-blind, controlled, prospective studies. Open Heart. 2015;2:e000220.
- Moyé LA, Pfeffer MA, Wun CC, et al. Uniformity of captopril benefit in the SAVE Study: subgroup analysis. Survival and Ventricular Enlargement Study. Eur Heart J. 1994;15(Suppl.B):2–8.
- Kostis JB. The effect of enalapril on mortal and morbid events in patients with hypertension and left ventricular dysfunction. Am J Hypertens. 1995;8:909–914.
- Yoshiyama M, Kamimori K, Shimada Y, et al. Left ventricular remodeling after myocardial infarction in antecedent hypertensive patients. Hypertens Res. 2005;28:293–299.
- Lang CC, Struthers AD. Targeting the renin-angiotensin-aldosterone system in heart failure. Nat Rev Cardiol. 2013;10:125–34.
- Rossini R, Senni M, Musumeci G, et al. Prevention of left ventricular remodelling after acute myocardial infarction: an update. Recent Pat Cardiovasc Drug Discov. 2010;5:196–207.
- Gajarsa JJ, Kloner RA. Left ventricular remodeling in the post-infarction heart: a review of cellular, molecular mechanisms, and therapeutic modalities. Heart Fail Rev. 2011;16:13–21.
- Yang JH, Hahn JY, Song YB, et al. Angiotensin receptor blocker in patients with ST segment elevation myocardial infarction with preserved left ventricular systolic function: prospective cohort study. BMJ. 2014;349:g6650.
- Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC), Steg PG, James SK, Atar D, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569–2619.
- Hansen ML, Gislason GH, Køber L, et al. Different angiotensin-converting enzyme inhibitors have similar clinical efficacy after myocardial infarction. Br J Clin Pharmacol. 2008;65:217–23.
- Cacciatore F, Bruzzese G, Vitale DF, et al. Effects of ACE inhibition on circulating endothelial progenitor cells, vascular damage, and oxidative stress in hypertensive patients. Eur J Clin Pharmacol. 2011;67:877–883.
- Napoli C, Bruzzese G, Ignarro LJ, et al. Long-term treatment with sulfhydryl angiotensin-converting enzyme inhibition reduces carotid intima-media thickening and improves the nitric oxide/oxidative stress pathways in newly diagnosed patients with mild to moderate primary hypertension. Am Heart J. 2008;156:1154.
- Omboni S, Malacco E, Parati G. Zofenopril plus hydrochlorothiazide fixed combination in the treatment of hypertension and associated clinical conditions. Cardiovasc Ther. 2009;27:275–288.
- Frascarelli S, Ghelardoni S, Ronca-Testoni S, et al. Cardioprotective effect of zofenopril in perfused rat heart subjected to ischemia and reperfusion. J Cardiovasc Pharmacol. 2004;43:294–299.
- Pasini AF, Garbin U, Nava MC, et al. Effect of sulfhydryl and non-sulfhydryl angiotensin-converting enzyme inhibitors on endothelial function in essential hypertensive patients. Am J Hypertens. 2007;20:443–450.
- Przyklenk K, Kloner RA. "Cardioprotection" by ACE-inhibitors in acute myocardial ischemia and infarction? Basic Res Cardiol. 1993;88(Suppl.1):139–154.