Abstract
Purpose: The aim of this observational cohort study was to investigate blood pressure level and the possibility to reach target blood pressure during concomitant use of NSAID in hypertensive patients.
Materials and methods: From the Swedish primary care cardiovascular database (SPCCD) a cohort of 5463 patients (2007 to 2008) with at least one prescription of NSAID dispensed 6 months prior to the last blood pressure measurement were included. Clinical data were extracted from computerized medical records and linked to the Prescribed Drug Register. Multivariable logistic regression models were used for analysis.
Results: Patients with NSAID usage were younger, more often female, with lower creatinine concentrations, more musculoskeletal diagnosis and less cardiovascular comorbidity compared to patients without dispensed NSAID (p < .0001 for all). Regular dose of NSAID was not associated with a decreased possibility to reach target blood pressure. A correlation between the dose of naproxen and an increase in SBP of 7 mm Hg was found. Impairment in renal function did not influence the association between blood pressure control and NSAID (p = .27).
Conclusion: In hypertensive patients with concomitant use of NSAID the chance to reach target blood pressure was not impaired. In intermediate and frequent users of NSAID there was a dose response relation with naproxen and SBP which was not found in diclofenac and ibuprofen.
Introduction
Approximately 30% of the patients in primary health care in Sweden have a pain disorder, and the majority are women [Citation1,Citation2]. Hypertension is similarly a common condition, and affects about 27% of the population, equally distributed in women and men [Citation3]. Approximately one fourth of elderly (>65 years) hypertensive patients treated in primary health care are concomitantly treated for arthrosis [Citation4]. Pain in arthrosis is the most common cause of long term use of non-steroidal anti-inflammatory drugs (NSAID) [Citation5]. Further, we know that 12–15% of elderly patients given NSAIDs are also prescribed an antihypertensive agent [Citation6].
NSAID are known to increase major cardiovascular events and upper gastrointestinal complications [Citation7]. Prostacyclin is an antithrombotic hormone, which is dependent on cycklooxygenase (COX) enzymes to convert arachnoidonic acid into prostaglandin H2. Inhibition of cyklooxygenase-1 and 2 (COX-1 and 2) explains the therapeutic effects of both NSAID and COX-2 selective drugs (coxibs). The prothrombotic effects have been attributed to the degree of COX-2 selectivity, where naproxen has been shown to confer the lowest and diclofenac and rofecoxib the highest risk. Ibuprofen has been placed in an intermediate risk position [Citation8]. NSAID interacts with the renin-angiotensin system and can influence renal function by reducing renal blood flow and glomerular filtration rate with important effects on blood pressure control. Also, NSAID have direct effects on prostaglandin turnover with effects on renal function and fluid balance. These effects of NSAID can lead to sodium retention and increase blood pressure, a major risk factor of cardiovascular disease [Citation9]. Thus, NSAID may interfere with the blood pressure lowering effect of many antihypertensive drug classes, possibly except calcium channel blockers [Citation10–12].
Two meta-analyses have shown that concomitant use of NSAID increase blood pressure, in particular in patients with hypertension [Citation13,Citation14]. However, recent international guidelines does not provide any recommendations regarding the use of NSAID in hypertensive patients [Citation15]. Nevertheless, the use of NSAID is often attributed as a possible explanation for resistant hypertension [Citation16].
The objective of this study was to investigate if concomitant use of NSAID in female and male hypertensive patients is associated with a lower possibility to reach target blood pressure. Second, we wanted to study if there was a dose response relation between commonly used NSAID and blood pressure level. Finally we aimed to investigate the effect of NSAID on blood pressure control with respect to renal function.
Methods
Study design
This was a cross-sectional study analysing the association between NSAID utilization and blood pressure levels and control. Data was collected from the Swedish primary care cardiovascular database (SPCCD) which includes 74,751 individuals 30 years and older in primary health care diagnosed with hypertension during January the 1st 2001 to December the 31st 2008. Computerized medical records (Profdoc Journal III, Profdoc AB, Uppsala, Sweden) from 48 primary health care centers were included. Demographic information, part of the free text (blood pressure), lifestyle information (height, weight, smoking status), comorbidity (by ICD–10 codes: diabetes mellitus, ischemic heart disease, heart failure, atrial fibrillation/flutter, cerebrovascular disease), and biochemistry (creatinine, fasting glucose, total cholesterol, HDL and LDL cholesterol, and triglycerides) were extracted from the medical records by a computerised tool, as described earlier [Citation17]. Estimated glomerular filtration rate was calculated by the CKD-EPI formula [Citation18]. Patients were classified into classes of chronic kidney disease 1–5 according to a glomerular filtration rate of >90, 60–89, 30–59, 15–29, and <15 ml/min per 1.73 m2; the two groups with the lowest values were analysed together due to the small numbers. Blood pressure was measured according to the national standards in the seated or supine position by either a physician or a nurse using either oscillometric or auscultatoric methods of measurement.
In the SPCCD clinical data is linked to five population based registers: the Prescribed Drug Register [Citation19], the National Patient Register including all hospitalizations and outpatient consultations in hospitals [Citation20], the Cause of Death Register [Citation21], the Census Registers, and the Swedish Register of Education [Citation22], using the personal identity number assigned to each Swedish resident [Citation23]. The details of SPCCD have been described elsewhere [Citation17].
The Regional Ethical Review Board in Gothenburg approved of the study.
For the purpose of this specific investigation we included a cohort of patients from the SPCCD who had at least one blood pressure measurement recorded between January 1st 2007, and December 31st, 2008. The date of the first blood pressure measurement during the study period was set to the index date. The patients were all diagnosed with hypertension before January 1st, 2007, to exclude patients with new onset of hypertension. Comorbidity, both cardiovascular and musculoskeletal, was obtained from relevant registers before the index date. Patients with at least one dispensed prescription of NSAID six months prior to the last blood pressure measurement made in 2007 or 2008 were included in the group of NSAID users. Patients who deceased during the study period (801 men and 883 women) were excluded from the analysis. Also patients in whom the dosage text on the prescription could not be interpreted or with automated multi-dose dispensed drugs (which lacked dosage instructions) were excluded.
The last blood pressure measurement during the study period was used in the analysis. This blood pressure has previously been shown to correlate with the mean value of the three last blood pressure measurements [Citation24]. Target blood pressure was set to <140/90 mm Hg for all patients.
Patient characteristics, comorbidity and antihypertensive therapy
Patient characteristics included age, gender, smoking, all blood pressure measurements, comorbidity, and laboratory analyses. Cardiovascular comorbidity was defined as a diagnosis of (with corresponding ICD–10 codes) atrial fibrillation/flutter (I48), heart failure (I50), diabetes mellitus (E10–11), ischemic heart disease (I20–25) or cerebrovascular disease (I60–69,G45) documented in the medical records and/or the National Patient Register. Antihypertensive drug classes assessed (with anatomic therapeutic chemical classification system (ATC) codes) were angiotensin converting enzyme inhibitors (ACEI) including fixed combinations with thiazides (C09A and C09B), angiotensin receptor blockers (ARB) including fixed combinations with thiazides (C09C and C09D), beta receptor blockers (C07), calcium channel blockers (C08 and CO7FB), thiazide like diuretics and amiloride (in the following denoted as thiazides; C03AA, C03AB, C03BA, C03EA), loop diuretics (C03CA), aldosterone antagonists (C03DA, C03DB), and other (which includes clonidine, moxonidine, doxazosin, and hydralazine; C02AC, C02CA, C02DB). Fixed drug combinations (eg, combinations of a diuretic and an ACEI or ARB, and the combination of a beta receptor blocker and a calcium channel blocker) were counted separate in their respective groups. All antihypertensive drugs recorded in the Prescribed Drug Register from January 1st, 2007, until December 31st, 2008 were included in the analysis. No analysis for possible changes in antihypertensive therapy during the study period was performed. Musculoskeletal disease was defined as a diagnosis in the ICD-10 classification of arthropathies (M00–M25), systemic connective tissue disorders (M30–M36), dorsopathies (M40–M54), soft tissue disorders (M60–M79), osteopathies and chondropathies (M80–M94), other disorders of the musculoskeletal system and connective tissue (M95–M99) in the National patient register before the index date. Data from the Swedish Register of Education was collected in 2005 and 2010. Educational level was divided in three categories: ≤9 years corresponding to primary educational level, 10–12 years corresponding to secondary school, and >12 years of education corresponding to college or university. Country of birth was obtained from the Census register in 2005 and 2008. Country of birth was grouped as follows; Sweden, Europe (excluding Sweden), and world (including Africa, North America, South America, Asia, Oceania and former Soviet Union, excluding Europe and Sweden).
NSAID
The NSAID used in Sweden during the study period included the following drug classes based on the ATC codes; acetic acid derivates (M01AB), oxicams (M01AC), propionic acid derivates (M01AE), and coxibs (M01AH). The group butylpyrazolidines (M01AA) and fenamates (M01AG) have no registered drugs in Sweden. The group “other antiinflammatory and anti-rheumatic agents, non-steroids” (M01AX) was excluded from the analysis to exclude glucosamine. To examine the association between frequent users and sporadic users of NSAID and blood pressure control the proportion of days covered (PDC) with prescription with NSAID 180 days prior to the latest blood pressure measurement was calculated. PDC, a measurement for adherence, was calculated with the total number of days of supply dispensed during a specified observation period divided by the number of days in the patients observation period, multiplied by 100 to obtain percent [Citation25]. The dosage was extracted from the text by pattern matching and based on the dosage and the text of the prescription during 180 days prior to the latest blood pressure measurement – index blood pressure. We considered a PDC <50%, 50–80%, and >80% to represent sporadic users, intermediate users, and frequent users. To analyse a possible dose-response association between the three most commonly used NSAID (diclofenac, ibuprofen and naproxen) and SBP, a calculation of daily dose (in mg) was performed based on the dosage text, the dispensed number of tablets during 180 days prior to the blood pressure measurement. In these analysis patients with a PDC of 50% or more were included to exclude sporadic users.
Statistics
Data are presented as mean values ± SD or with 95% confidence intervals (CI), as appropriate. Comparisons of groups were made using the Chi2 test for categorical variables and the Student’s t-test for continuous variables. Odds ratios were calculated and trends were analysed using a multivariable logistic regression models. Covariates adjusted for in the logistic regression models (age, gender, smoking, antihypertensive drug class, cardiovascular comorbidity, education, ethnicity and musculoskeletal disease) were tested and found significant (p < .05) in univariate regression analyses. There were missing data on smoking in 57% (21,900 patients) of the study cohort. The group with missing data on smoking was tested and found to have a significant impact in the models; thus a group with missing data for smoking was created and adjusted for in the models. Missing data on educational level in 1300 patients were adjusted for in a similar way. The associations between increase in SBP and doses in naproxen, diclofenac and ibuprofen were analysed using cubic restricted splines in regression models adjusted for covariates. All analyses were conducted using SAS version 9.3 (SAS Institute, Cary, North Carolina, USA). A two-tailed probability value (p) < .05 was considered significant.
Results
In all 40,825 patients had one or more blood pressure measurements during 2007 and 2008. However, 457 patients with unclear dosage text and 2117 individuals with prescribed automated multi-dose drug dispensing were excluded. This rendered a study cohort of 38,251 patients, and 5463 had at least one prescription of NSAID dispensed 6 months prior to the last blood pressure measurement.
The majority of patients with NSAID were slightly younger, of female gender, with lower creatinine concentrations and more often with a diagnosis of musculoskeletal disease but without cardiovascular comorbidity (). Women in general had higher SBP and lower DBP (). The majority of NSAID users were sporadic users (PDC <50%; 2485 women and 1633 men), followed by intermediate users (PDC 50–80%; 505 women and 292 men) and frequent users (PDC >80%; 370 women and 178 men). In a logistic regression model when analysing women and men separately, only men with a PDC of 50–80% achieved target blood pressure to a lesser degree ().
Figure 1. Box and whiskers plot with the top and bottom boundaries of the box representing interquartile range and the line in the box representing the mean value. The whiskers show the upper and lower extremes, excluding outliers. PDC, proportion of days covered with NSAID treatment 180 days prior to the last blood pressure measurement. Significant differences between men and women are shown as *p < .05, and ***p < .001.
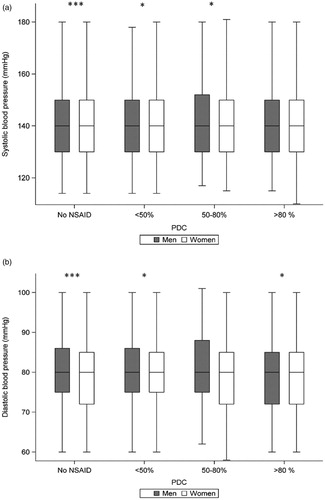
Figure 2. Achieved target blood pressure (<140/90 mm Hg) in patients without NSAID (reference group) and in patients with NSAID, divided into different PDC groups according to gender Presented as odds ratio by a logistic regression model adjusted for age, gender, smoking, antihypertensive drug class, cardiovascular comorbidity, education, ethnicity, and musculoskeletal disease. BP: blood pressure; PDC: proportion of days covered with NSAID treatment 180 days prior to the last blood pressure measurement.
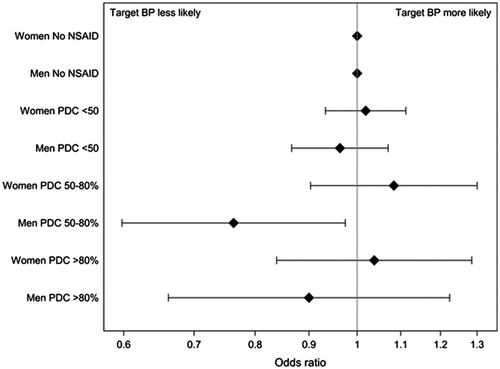
Table 1. Patients characteristics of 38,251 patients with and without a prescription of NSAID 180 days prior to the last blood pressure measurement.
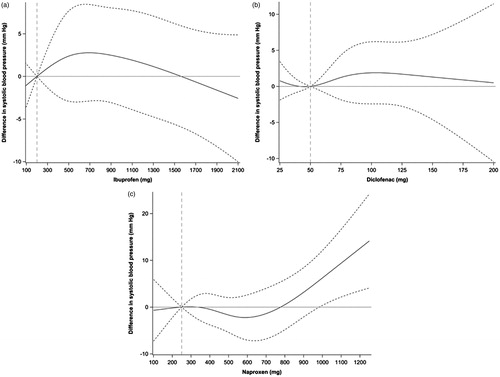
The three most commonly prescribed NSAID were naproxen, diclofenac, and ibuprofen. When analysing the association between dose and SBP in patients with a PDC >50% prior to the last blood pressure measurement, we identified 475 patients with diclofenac, 286 with ibuprofen, and 236 with naproxen. A regression model adjusted for covariates showed an association between the dose of naproxen and an increase in SBP (when comparing the reference dose of 250 mg with higher doses) of 7 mm Hg with the maximum dose of 1000 mg naproxen (). However, as illustrated in the association was not linear and the number of individuals was few. In patients with ibuprofen and diclofenac the associations between dose and SBP were weak when comparing reference doses of 50 mg diclofenac and 200 mg ibuprofen with higher daily doses ().
Figure 3. (a–c) Regression models presented with a spline curve with upper and lower 95% CI (dashed line), analysing changes in SBP from a reference daily dose (naproxen 250 mg, diclofenac 50 mg, and ibuprofen 200 mg). Data for naproxen (a; n = 256), diclofenac (b; n = 475), and ibuprofen (c; n = 286) were adjusted for age, gender, smoking, number of antihypertensive drugs, cardiovascular comorbidity, education, ethnicity, and musculoskeletal disease.
In the total cohort the proportion of patients with controlled blood pressure decreased with lower estimated glomerular filtration rate (Chi2 22.9, p < .0001). This relation appeared consistent in patients with and without NSAID, and across different classes of NSAID (). There was no difference in control rates between NSAID and no NSAID groups across chronic kidney disease classes (Chi2 1.18, p = .28) and no interaction between blood pressure control across chronic kidney disease classes and NSAID use (Chi2 1.21, p = .27) ().
Table 2. Controlled blood pressure in relation to treatment with NSAID by renal function.
Discussion
This large cross-sectional study showed no association between the possibility to reach target blood pressure and the use of NSAID, irrespective of the proportion of days covered by NSAID. Results were similar in women and men with the exception of men with a medium use of NSAID where target blood pressure was achieved to a lesser degree. A dose related increase in SBP with high dosage of naproxen was identified, but the use of NSAID did not influence the interaction between blood pressure control and renal function.
To the best of our knowledge no prior study has investigated the ability to reach target blood pressure in the elderly hypertensive patients using NSAID in clinical practice. In our study the mean age of the patients receiving NSAID was 67 years, and the proportion of patients prescribed NSAID was 17%. This is in line with results from the United States where 12–15% of the elderly patients are prescribed concomitant antihypertensive and NSAID drugs [Citation6]. In a younger population the proportion of NSAID users among hypertensive patients is higher, approximately 30% [Citation26]. Few randomized controlled trials regarding blood pressure levels and use of NSAID have been performed among older patients, and often these investigations include few subjects and the study duration is short. Data from one case control trial shows that the use of NSAIDs increase the risk of initiating antihypertensive therapy in the elderly (>65 years) [Citation27]. Thus, our findings that target blood pressure could be achieved despite concomitant treatment with NSAID are encouraging, and hypertensive patients in need of anti-inflammatory drugs do not á priori need to be discouraged to use NSAID in recommended doses if blood pressure levels are closely monitored. However, caution with NSAID should be made in patients with a history of heart failure or other cardiovascular disease given the increased risk of cardiovascular events [Citation7].
Among women, who were a majority of the patients no difference in achieved target blood pressure was seen in relation to PDC. However, we observed that men with a PDC of 50–80% were less likely to achieve target blood pressure. The explanation for this is not clear. The groups were small when analysing women and men separately and we cannot exclude that these results regarding gender differences are attributed to a chance finding.
Randomized control trials performed among younger subjects show contradictory results regarding the use of NSAID and blood pressure [Citation28]. Two meta-analyses in younger patients (mean age 46 and 48 years) showed an increase in blood pressure of 1.1 mm Hg in normotensive individuals and 3.3 mm Hg in hypertensive individuals [Citation14], and a 5.4 mm Hg increase in patients with controlled hypertension and long-term NSAID therapy [Citation13]. In these studies a difference in blood pressure increase by the type of NSAID was evident, where indomethacin (no longer registered in Sweden), naproxen, and piroxicam proposed the greatest increase in blood pressure. Accordingly, in our study naproxen was associated with a dose dependent increase in SBP (7 mm Hg for the 1000 mg dose of naproxen) while the effects of diclofenac and ibuprofen were small.
As expected, we observed a reduced blood pressure control with progressive impairment of renal function. More important, however, the proportion of patients with achieved blood pressure control were similar for NSAID and no NSAID groups across all chronic kidney disease classes, and there was no interaction between blood pressure control across renal function and NSAID use. These results suggest that a standard use of NSAID in patients treated for hypertension does not need to cause any major problem regarding blood pressure control, also in patients with renal impairment.
Our results are of interest in the context of the somewhat discrepant findings that high doses of diclofenac and possibly ibuprofen propose the greatest risk of vascular events comparable to coxibs, whereas the risk with naproxen is less [Citation7]. Taken together, the results suggest that the use of ibuprofen in hypertensive patients have a marginal impact on SBP, and further propose an intermediate risk of thromboembolic events compared to diclofenac. Thus, in hypertensive patients in need of NSAID ibuprofen could be the most favourable drug.
There are some strengths of this study. All information was extracted directly from the patient’s medical records and relevant registers, which make the data largely unbiased. The study cohort represents a hypertensive cohort with a mean age of 67 years for patients with NSAID. Few studies have evaluated the use of NSAID and the ability to reach target blood pressure among elderly hypertensive patients. However, our study has some important limitations. Data originate from clinical patient data extracted directly from medical records. This renders some missing values on biochemistry, smoking, and educational level which we tried to eliminate by adjustment in the statistical models. Information about alcohol habits and salt intake, which might influence the results, was not available. Our study includes only prescribed and iterated NSAID and there is a large over the counter purchase of NSAID, which might influence the results. However, for the elderly and for the frequent users the majority of NSAID are prescribed [Citation29]. We did not consider possible changes in blood pressure medication during the study period which might influence our results. The study period was on the other hand limited to 6 months which ought to restrict the possible influence of changes in antihypertensive drug therapy. The cross-sectional design has limitations in its difficulties making causal inference. The interpretation of the role of different doses of NSAID and blood pressure should be made with caution as the number of patients in the subgroups was limited. Finally, the time frame was limited and we cannot rule out that the most vulnerable patients were already deceased, which may influence the results.
In conclusion, in the present study concomitant use of regular dose of NSAID in hypertensive patients is not associated with a decreased possibility to reach target blood pressure, and NSAID does not seem to influence blood pressure control in patients with impairment renal function. In intermediate and frequent users of NSAID there is a dose response relation with naproxen and SBP which is not found in usage of diclofenac and ibuprofen. Thus, in patients with hypertension in need of NSAID ibuprofen may be a preferred choice if an excessive risk of thromboembolic complications is taken into account.
Contribution
CL had the main responsibility for planning of the study, interpreting the data and writing the manuscript. LS was mainly responsible for the statistical analysis. JH introduced the idea of the manuscript. All authors took active part in planning of the study and reviewing the manuscript. Together with CL, TK and KM were active in finalizing the manuscript after interpreting the data. All authors approved the final version of the manuscript.
Acknowledgements
The SPCCD is endorsed by the Swedish Society for Hypertension, Stroke and Vascular Medicine. We acknowledge the important contribution of all participating primary health-care centers.
Disclosure statement
The authors have no conflict of interest to declare. T Kahan has research grants from Medtronic, Pfizer, and Amgen, all outside the submitted work.
Additional information
Funding
References
- Hasselstrom J, Liu-Palmgren J, Rasjo-Wraak G. Prevalence of pain in general practice. Eur J Pain. 2002;6:375–385.
- Gustavsson A, Bjorkman J, Ljungcrantz C, et al. Socio-economic burden of patients with a diagnosis related to chronic pain–register data of 840,000 Swedish patients. Eur J Pain. 2012;16:289–299.
- Lindholm LH, Carlberg B. Moderately elevated blood pressure. A systematic literature review. Vol. 1&2. Stockholm: The Swedish Council on Technology Assessment in Health Care 2004. SBU-rapport 170 = 1:1–514 and 170 = 2:1–248. 2004.
- Boeckxstaens P, Peersman W, Goubin G, et al. A practice-based analysis of combinations of diseases in patients aged 65 or older in primary care. BMC Fam Pract. 2014;15:159.
- Thompson PW, Tee L, McBride J, et al. Long-term NSAID use in primary care: changes over a decade and NICE risk factors for gastrointestinal adverse events. Rheumatology (Oxford). 2005;44:1308–1310.
- Johnson AG, Simons LA, Simons J, et al. Non-steroidal anti-inflammatory drugs and hypertension in the elderly: a community-based cross-sectional study. Br J Clin Pharmacol. 1993;35:455–459.
- Coxib and traditional NSAID Trialists' (CNT) Collaboration, Bhala N, Emberson J, Merhi A, et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382:769–779.
- McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA. 2006;296:1633–1644.
- Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260.
- Conlin PR, Moore TJ, Swartz SL, et al. Effect of indomethacin on blood pressure lowering by captopril and losartan in hypertensive patients. Hypertension. 2000;36:461–465.
- Bakris GL, Kern SR. Renal dysfunction resulting from NSAIDs. Am Fam Physician. 1989;40:199–204.
- Ishiguro C, Fujita T, Omori T, et al. Assessing the effects of non-steroidal anti-inflammatory drugs on antihypertensive drug therapy using post-marketing surveillance database. J Epidemiol. 2008;18:119–124.
- Johnson AG, Nguyen TV, Day RO. Do nonsteroidal anti-inflammatory drugs affect blood pressure? A meta-analysis. Ann Intern Med. 1994;121:289–300.
- Pope JE, Anderson JJ, Felson DT. A meta-analysis of the effects of nonsteroidal anti-inflammatory drugs on blood pressure. Arch Intern Med. 1993;153:477–484.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–1357.
- Sarafidis PA, Bakris GL. Resistant hypertension: an overview of evaluation and treatment. J Am Coll Cardiol. 2008;52:1749–1757.
- Hasselstrom J, Zarrinkoub R, Holmquist C, et al. The Swedish Primary Care Cardiovascular Database (SPCCD): 74 751 hypertensive primary care patients. Blood Press. 2014;23:116–125
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612.
- Wettermark B, Hammar N, Fored CM, et al. The new Swedish Prescribed Drug Register: opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16:726–735.
- Ludvigsson J, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450.
- Johansson LA, Westerling R. Comparing Swedish hospital discharge records with death certificates: implications for mortality statistics. Int J Epidemiol. 2000;29:495–502.
- Weitoft GR, Rosen M, Ericsson O, et al. Education and drug use in Sweden–a nationwide register-based study. Pharmacoepidemiol Drug Saf. 2008;17:1020–1028.
- Ludvigsson J, Otterblad-Olausson P, Pettersson B, et al. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24:659–667.
- Hasselstrom J, Zarrinkoub R, Holmquist C, et al. The Swedish Primary Care Cardiovascular Database (SPCCD): 74 751 hypertensive primary care patients. Blood Press. 2014;23:116–125.
- Raebel MA, Schmittdiel J, Karter AJ, et al. Standardizing terminology and definitions of medication adherence and persistence in research employing electronic databases. Med Care. 2013;51:S11–S21.
- Mazzaglia G, Ambrosioni E, Alacqua M, et al. Adherence to antihypertensive medications and cardiovascular morbidity among newly diagnosed hypertensive patients. Circulation. 2009;120:1598–1605.
- Gurwitz JH, Avorn J, Bohn RL, et al. Initiation of antihypertensive treatment during nonsteroidal anti-inflammatory drug therapy. JAMA. 1994;272:781–786.
- Johnson AG. NSAIDs and blood pressure. Clinical importance for older patients. Drugs Aging. 1998;12:17–27.
- Duong M, Salvo F, Pariente A, et al. Usage patterns of ‘over-the-counter’ vs. prescription-strength nonsteroidal anti-inflammatory drugs in France. Br J Clin Pharmacol. 2014;77:887–895.
