Abstract
Objective: Previous trials of catheter-based renal-artery denervation (RDN) as treatment modality in resistant hypertension (rHT) generated unconvincing results. In the Investigator-Steered Project on Intravascular Denervation for Management of Treatment-Resistant Hypertension (INSPiRED; NCT01505010), we optimized selection and management of rHT patients.
Methods: With ethical clearance to randomize 18 patients, three Belgian hypertension centers screened 29 rHT patients on treatment with ≥3 drugs, of whom 17 after optimization of treatment (age <70 years; systolic/diastolic office blood pressure (BP) ≥ 140/90 mm Hg; 24-h BP ≥130/80 mm Hg; glomerular filtration rate [eGFR] ≥ 45 mL/min/1.73 m2; body mass index <40kg/m2) were randomized and 15 were analyzed 6 months later, while medical treatment was continued (n = 9) or combined with RDN by the EnligHTN™ multi-electrode system (n = 6).
Results: The baseline-adjusted between-group differences amounted to 19.5/10.4 mm Hg (change in control vs. intervention group, +7.6/+2.2 vs. −11.9/−8.2 mm Hg; P = .088) for office BP, 22.4/13.1 mm Hg (+0.7/+0.3 vs. −21.7/−12.8; mm Hg; P ≤ .049) for 24-h BP, the primary efficacy endpoint, and 2.5 mL/min/1.73 m2 (+1.5 vs. −1.1 mL/min/1.73 m2; P = .86) for eGFR, the primary safety endpoint. At 6 month, ECG voltages and the number of prescribed drugs (P ≤ .036) were lower in RDN patients, but quality of life and adherence, captured by questionnaire and urine analysis were similar in both groups. Changes in BP and adherence were unrelated. No major complications occurred.
Conclusions: The INSPiRED pilot suggests that RDN with the EnligHTN™ system is effective and safe and generated insights useful for the design of future RDN trials.
Introduction
Resistant hypertension is an office blood pressure of at least 140 mm Hg systolic or 90 mm Hg diastolic on treatment with three antihypertensive drug classes at maximal doses, including a diuretic [Citation1]. In 2009, Krum and colleagues reported a non-randomized proof-of-concept study (SYMPLICITY HTN-1) [Citation2], showing that percutaneous radiofrequency catheter-based renal sympathetic nervous denervation (RDN) was feasible, effective and safe in patients with treatment-resistant hypertension. Subsequently, the SYMPLICITY HTN-2 study [Citation3], an open-label randomized clinical trial, reported an impressive reduction in office blood pressure 6 months after renal denervation, using the SYMPLICITY™ radiofrequency catheter in hypertensive patients unresponsive to multiple drugs. However, in 2014, the properly powered SYMPLICITY HTN-3 trial [Citation4], which had a randomized design with a sham arm, as requested by the Food and Drug Administration, failed to reach its primary efficacy endpoint, a reduction in office systolic blood pressure 6 months after RDN. A subsequent meta-analysis of 985 patients enrolled in seven randomized clinical trials confirmed that RDN with the single electrode SYMPLICITY™ radiofrequency catheter did not decrease blood pressure [Citation5], although a minority of patients experienced benefit in terms of blood pressure control [Citation6].
SYMPLICITY HTN-3 [Citation4] shattered the seemingly unlimited prospects of a large highly profitable market and stalled most research on RDN. While remaining convinced of the potential of RDN as a treatment modality in properly selected patients with resistant hypertension [Citation7], we designed the Investigator-Steered Project on Intravascular Renal Denervation for Management of Drug-Resistant Hypertension (INSPiRED; NCT01505010) [Citation8]. In view of the null results of SYMPLICITY HTN-3, [Citation4] we received ethical clearance for a pilot study with 18 randomized patients to be followed up for 6 months after randomization. The objective of this article is to report the results of the INSPiRED pilot trial [Citation8] and to set the stage for a full trial.
Methods
Study design
INSPiRED complies with the declaration of Helsinki [Citation9]. The trial received ethical approval from the competent Institutional Review Boards of the three participating clinical centers in Belgium, including the University Hospitals Leuven (No. B3222014197090). The rationale and design of the INSPiRED study have been published previously [Citation8] and are fully described in the Expanded Methods available in the online-only Data Supplement.
In short, INSPiRED is a multicenter randomized controlled trial, in which treatment-resistant hypertensive patients are randomly allocated to usual medical treatment (control group) or usual medical treatment plus RDN (intervention group). The trial consists of six consecutive stages. First, at a screening visit, each patient’s eligibility was checked according to predefined inclusion and exclusion criteria [Citation8], and patients were invited to provide informed written consent. Second, during a run-in period of flexible duration, usually lasting 1 to 3 months, hypertension specialists optimized medical treatment according to the AB/CD algorithm [Citation8,Citation10], while recording office and 24-h ambulatory blood pressure. Concurrently, additional examinations, as indicated in each case, served to exclude secondary hypertension. The entry characteristics of patients were determined at the last run-in visit. Next, after stratification by center and age (20–49 vs. 50–69 years) and after quality control of the data provided to the Study Coordinating Center, eligible patients were randomized in equal proportions to continuation of optimized medical treatment alone (control group) or combined with RDN (intervention group). Patients were randomized at the Studies Coordinating Centre by means of a computerized random function with block size restriction to ensure balanced allocation of patients across centers and the four strata. Subsequently, during stage 4, patients were followed up 1, 3 and 6 months with an optional visit at 2 months. To maintain the best possible blood pressure control, adjustment of antihypertensive drug treatment after randomization remained possible in both groups. Finally, patients randomized to control could cross-over at 6 months and all patients will remain in follow-up for 3 years. However, data beyond the 6-month follow-up fall beyond the scope of the current report on the pilot study.
Selection of patients
Eligible patients have essential hypertension, are younger than 70 years, and have a glomerular filtration rate estimated from serum creatinine by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [Citation11] not less than 60 mL/min/m2 at screening and per amendment to the trial protocol (No. B3222014197090; 23 December 2014) not less than 45 mL/min/m2 at the last run-in visit. Optimizing treatment involves the prescription of three or more antihypertensive medications from different drug classes, preferably including a diuretic [Citation8]. An aldosterone receptor antagonist and a β-blocker should have been tried, unless contra-indicated. On optimized treatment, office blood pressure should be at least 140 mm Hg systolic or 90 mm Hg diastolic and the 24-h blood pressure should not drop below 130 mm Hg systolic and 80 mm Hg diastolic. The main renal arteries should have a diameter of at least 4 mm and a length not less than 20 mm [Citation8].
Endpoints
The primary endpoints for efficacy and safety are the baseline-adjusted between-group differences in the 24-h systolic ambulatory blood pressure and in eGFR, respectively. The 24-h ambulatory blood pressure recordings stayed unedited except for readings marked by an error code and were blindly decoded by staff not involved in the trial. Office and ambulatory blood pressures are measured according to current European guidelines [Citation12,Citation13], using an appropriate cuff size. For office blood pressure, the automated and validated [Citation14] Omron HEM-907 device (Omron Health Care, Kyoto, Japan) was used. The in-office blood pressure is the average of three consecutive measurements obtained after the patients had rested for 5 minutes in the sitting position. Standing blood pressure measured immediately thereafter is the average of two readings. Orthostatic blood pressure change is the difference of sitting minus standing blood pressure. For ambulatory blood pressure monitoring, portable and properly validated oscillometric devices (Mobil O Graph, I.E.M GmbH, Stolberg, Germany [Citation15]; ABPM-04, Meditech Ltd., Budapest, Hungary [Citation16]) were programmed to obtain readings at intervals ranging from 15 to 30 minutes during daytime and from 30 to 60 minutes during nighttime. Standard 12-lead electrocardiograms were recorded at a speed of 25 mm/sec with the calibration routinely set at 1 mV per cm. The ECG software allowed exporting the digital ECG measurements into the SAS statistical software package with an accuracy of 0.1 mV for voltages and 1 ms for duration indexes, respectively.
Drug treatment
All patients received advice on lifestyle. To optimize blood-pressure lowering drug treatment, hypertension specialists applied current guidelines [Citation10,Citation17]. The treatment strategies were standardized as follows: (i) by using combinations of antihypertensive drugs with different mode of action in line with the AB/CD algorithm [Citation8,Citation10]; (ii) by using antihypertensive agents with a long duration of action, so-called forgiving drugs [Citation18]; (iii) by enhancing adherence via reduction of the pill load and by prescription of single-pill combination tablets [Citation19], including two or three antihypertensive agents in adjustable doses; (iv) for each antihypertensive drug, the highest dose that did not produce side-effects was prescribed; (v) antihypertensive drug combinations preferably included a diuretic; (vi) and if not contra-indicated, use of aldosterone receptor antagonists and β-blockers should at least have been attempted.
Other procedures
With patients in supine, left or right decubitus, experienced ultrasonographers obtained renal gray scale images. Renal length was the largest longitudinal distance in the sagittal plane. The default for imaging of the renal arteries was multi-detector computerized tomographic angiography (aCT) [Citation20]. Contrast-enhanced magnetic resonance imaging (MRI) was used as an alternative in case of iodine allergy or a contraindication for aCT. RDN was performed using the EnligHTN™ multi-electrode denervation system (St Jude Medical, Saint Paul, MN) [Citation21]. This system makes it possible to perform four ablations simultaneously. A minimum of 8 and a maximum of 12 ablations were delivered from the distal main renal artery bifurcation to the ostium. The eight-item Morisky Medication Adherence Scale (MMAS-8) was used to assess adherence to antihypertensive drugs [Citation22,Citation23]. Scores of 8, 7-6 and less than 6 signify high, medium and low adherence [Citation22,Citation23]. In addition, we applied a liquid chromatography–mass spectrometric method that allows antihypertensive drugs or their metabolites to be detected in a qualitative manner in a 10-mL urine sample [Citation24,Citation25]. Quality of life was assessed by means of the EQ-5D-5L questionnaire, a standardized instrument to measure health status developed by the EuroQoL Group (www.euroqol.org) [Citation26]. In addition, a visual analogue scale assesses the patient’s self-rated health on a scale ranging from 0 to 100 [Citation26].
Statistical analysis
For database management and statistical analysis, we used SAS software version 9.4. (SAS Institute, Cary, NC). The Studies Coordinating Centre in Leuven was in charge of the management of the trial, the stratification and randomization of patients, database management and statistical analyses. We reported the central tendency and spread of the data as mean and standard deviation for normally distributed data and as median and interquartile range (IQR) for results with a non-normal distribution. For comparison of means, medians and proportions, we applied Student t test, Wilcoxon rank sum test and Fisher exact test, respectively. Statistical significance was a P-value less than 0.05 on two-sided tests. We applied the Wilcoxon rank sum test to test the between-group differences in the changes from baseline to 6 months in blood pressure and eGFR. We also used mixed models to compare blood pressure changes between randomized groups at 1, 3 and 6 months, while adjusting for the baseline blood pressure.
Results
Patient recruitment
From 28 March 2014 until 11 May 2016, three Belgian hypertension expert centers screened 29 treatment-resistant patients, of whom 4 did not meet the criteria to enter the run-in period, because of renal artery stenosis, being on the waiting list for elective surgery, normalization of the 24-h ambulatory blood pressure, or failure to provide informed consent (Figure S1 available in the online-only Data Supplement). Of the 25 patients entering the run-in period, 8 were not eligible for randomization, because of fibromuscular dysplasia, normalization of the 24-h blood pressure, withdrawal of consent, psychiatric illness, or loss to follow-up. Of the remaining 17 patients, 9 were randomized to control and 8 to RDN. Of the 8 patients randomized to intervention, two did not undergo RDN, because one patient was lost to follow-up before RDN and one patients was diagnosed with fibromuscular dysplasia on intra-arterial angiography preparing for RDN. The number of patients statistically analyzed was therefore 9 and 6 in the control and intervention group, respectively (Figure S1).
Among the 15 randomized patients, 8 were female (53.3%), the median history of hypertension was 10 years (IQR, 5–16 years) and the median number of antihypertensive drugs taken at randomization was 4 (IQR, 3–5). Age ranged from 33.6 to 62.3 years, body mass index from 22.4 to 37.9 kg/m2, and eGFR from 46.2 to 114.5 mL/min/1.73 m2; mean (±SD) values were 48.1 ± 9.3 years, 30.4 ± 4.6 kg/m2, and 85.4 ± 20.7 mL/min/1.73m2, respectively. There were no significant between-group differences in the baseline characteristics (), including office or ambulatory blood pressures (), ECG voltages (), adherence as assessed by MMAS-8 or urine analysis and quality of life (Table S1).
Table 1. Characteristics of participants.
Table 2. Baseline-adjusted changes in blood pressure 6 months after randomization.
Table 3. Baseline-adjusted differences in ECG measurements.
Efficacy endpoints
For all patients, three sitting and two standing office blood pressure readings had to be obtained at baseline and at 6 months. For the sitting blood pressure, there were no missing values and none of the three consecutive sitting systolic or diastolic blood pressure readings were identical. For standing blood pressure, one patient had missing values and two consecutive standing systolic blood pressure readings were identical in a single patient. The median number of valid blood pressure readings obtained by ambulatory monitoring at baseline was 56 (IQR, 34–71; 5th–95th percentile interval [PI], 22–74) over 24 hours and 25 (IQR, 18–36; PI, 11–43) and 12 (IQR, 6–13; PI, 4–13) during day- and nighttime, respectively. At the 6 month follow-up visit, these numbers were 53 (IQR, 38–66; PI, 28–80), 23 (IQR, 17–32; PI, 13–42) and 12 (IQR, 7–13; PI, 5–13), respectively.
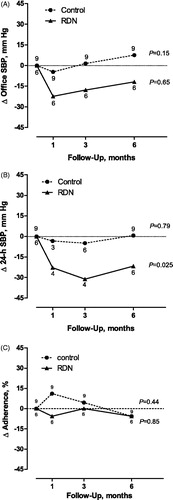
In the control group (), the baseline-adjusted within-group changes (follow-up at 6 months minus baseline) in office, 24-h, daytime and nighttime systolic/diastolic blood pressures averaged +7.6/+2.2 mm Hg, +0.7/+0.3 mm Hg, +0.8/-0.6 mm Hg and +1.4/+0.9 mm Hg; in the RDN group, these changes averaged −11.9/−8.2 mm Hg, −21.7/−12.8 mm Hg, −13.8/−7.1 mm Hg and −31.5/−21.1 mm Hg, respectively. illustrates that the decrease in systolic blood pressure was consistent in 5 of 6 RDN patients for office blood pressure and in all RDN patients for the 24-h blood pressure. Similar data for day- and nighttime blood pressure appear in Figure S2. Most of the blood pressure response to RDN was already achieved at 1 month and maintained up to 6 months (). At 6 months, the between-group baseline-adjusted differences in systolic blood pressure (control minus RDN) amounted 19.5 mm Hg (95% confidence interval [CI], −4.7 to 43.6 mm Hg; P = .088), 22.4 mm Hg (CI, 5.6 to 39.1 mm Hg; P = .018), 14.5 mm Hg (CI, −10.0 to 39.1 mm Hg; P = .22) and 32.9 mm Hg (CI, 12.2 to 53.5 mm Hg; P = .008) on office, 24-h, daytime and nighttime measurement (). The corresponding differences in diastolic blood pressure amounted to 10.4 mm Hg (CI, −5.1 to 26.0 mm Hg; P = .088), 13.1 mm Hg (CI, 0.4 to 25.7 mm Hg; P = .049), 6.5 mm Hg (CI, −8.4 to 21.4 mm Hg; P = .33) and 22.0 mm Hg (CI, 8.4 to 35.5 mm Hg; P = .008), respectively (). At the 6-month visit, no patient randomized to control reached office (<140/<90 mm Hg) or 24-h (<130/<80 mm Hg) normotension; in the intervention group 2 (40.0%) reached 24-h normotension and 1 (20.0%) both office and 24-h normotension.
Figure 1. Change (Δ) in office systolic blood pressure (A) and 24-h ambulatory systolic blood pressure (B) from baseline to the 6-months follow-up in individual patients randomized to control (n = 9) or renal denervation (RDN; n = 6). SBP indicates systolic blood pressure.
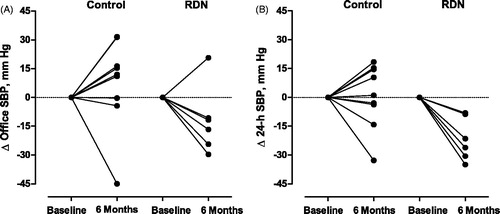
Figure 2. Time course of the change (Δ) in office systolic blood pressure (A), 24-h ambulatory systolic blood pressure (B) and adherence (C) from baseline until the 6-month follow-up. Adherence was expressed as the change in percentage of prescribed drugs detectable in urine. P-values were obtained from a mixed model assessing the within-group changes in blood pressure and adherence over follow-up time.
At baseline standing office systolic/diastolic blood pressure averaged 166.8 ± 26.2/100.4 ± 16.8 mm Hg in the control group and 173.8 ± 34.7/111.3 ± 17.3 mm Hg in the RDN group. At 6 months, these levels were 167.6 ± 16.6/101.6 ± 16.8 mm Hg and 164.2 ± 31.9/102.9 ± 18.6 mm Hg, respectively. The baseline-adjusted between-group differences were 10.4 mm Hg systolic (CI, −16.0 to 36.8 mm Hg; P = .30) and 7.0 mm Hg diastolic (CI, −6.3 to 20.3 mm Hg; P = .35). At 6 months the baseline-adjusted between-group difference in the orthostatic blood pressure change averaged −8.7 mm Hg systolic (CI, −18.4 to 1.1 mm Hg; P = .059) and −2.3 mm Hg diastolic (CI, −11.5 to 7.0 mm Hg; P = .47). One patient reported an in-hospital presyncope shortly after RDN, but otherwise no syncope occurred in either group. During follow-up, three patients in the control group and one in the RDN group reported accidental falls.
At 6 months, the baseline-adjusted between-group difference in the ECG indexes were 0.92 mV (CI, 0.42 to 1.43 mV; P = .002) for the Cornell voltage; 2.90 mV (CI, 1.12 to 4.66 mV; P = .005) for the Siegel total voltage, 84 mV × ms (CI, 18 to 149 mV × ms; P = .036) for the Cornell index and 274 mV × ms (CI, 26 to 523 mV × ms; P = .036) for the Siegel product. All patients were in sinus rhythm at baseline and during follow-up.
Imaging and RDN procedure
The renal arteries were imaged by aCT in 8 control and 6 RDN patients and by MRI in a single control patient. In the control group, the main renal arteries had a diameter of 5.2 ± 1.1 mm right and 5.5 ± 1.2 mm left. In the RDN group, the corresponding dimensions were 6.1 ± 2.2 mm right and 6.4 ± 1.7 mm left (P ≥ .44 for between-group differences). RDN was performed under local or general anesthesia in one and five patients, respectively. Via right femoral access, a sheet of 8 F in five patients and 6 F in one patient was pushed forward to the renal arteries. The median duration of the RDN procedure was 82.5 min (IQR, 56.3 to 97.5 min). In all patients at least two ablations sectors per artery (left and right), one distal and one proximal, were delineated. The number of complete ablations per zone ranged from 2 to 4. One RDN patient had bilateral accessory renal arteries, which were accessible for RDN and were denervated. Renal spasms occurred in four patients, but did not lead to persistent lesions.
Safety
Baseline values of serum creatinine and eGFR appear in . At 6 months, these renal indexes averaged 0.99 ± 0.35 mg/dL and 81.6 ± 24.1 mL/min/1.73 m2 in control patients and 0.86 ± 0.24 mg/dL and 92.4 ± 15.9 mL/min/1.73 m2 in the RDN group. The baseline-adjusted between-group differences at 6 months were −0.02 mg/dL (CI, −0.13 to 0.09 mg/dL; P = .89) for serum creatinine and 2.5 mL/min/1.73 m2 (CI, −7.0 to 12.1 mL/min/1.73 m2; P = .86) for eGFR. No acute procedural adverse events occurred. In one patient undergoing RDN, mild irregularities of the left renal artery were observed 6 months after RDN.
Adherence
Compared with baseline (), at 6 months, the median number of drugs prescribed per day remained unchanged in the control group (4.0 [IQR, 4.0 to 6.0]; P = .26), but decreased to 3.0 [IQR, 2.0 to 4.0]; P = .037) in the RDN group, resulting in a baseline-adjusted between-group difference of 1.7 (CI, 0.1 to 3.3; P = .032). Details on drug treatment by treatment group at the 6-month visit appear in Table S2. Compared with baseline (), the median MMAS-8 score at 6 months was similar in control (8.0; IQR, 8.0–8.0; P = .73) and RDN patients (7.9; IQR, 8.0-8.0; P = .93). Consequently, at 6 months, there was no baseline-adjusted between-group difference in the MMAS-8 score (−0.2; CI, −1.4 to 1.1; P = .74).
Analyses of urine samples detected intake of 45 antihypertensive drugs, which covered 94.9% of the prescribed drugs. In continuous analyses, we expressed adherence as the percentage of prescribed drugs that were detected in urine. At baseline, this proportion averaged 56.8 ± 38.6% in control patients and 62.3 ± 45.2% in the RDN group. At 6 months, these proportions remained unchanged in both groups (P ≥ .71), averaging 51.2 ± 42.4% and 56.7 ± 49.7%, respectively (). The baseline-adjusted between-group difference was 0.11% (CI, −59.7 to 59.9%; P = .89). The rank correlations between the changes in the 24-h systolic/diastolic blood pressure and adherence (continuous) from baseline to 6 months were 0.05/−0.07 (P ≥ .86) and 0.03/−0.09 (P ≥ .87) in the control and RDN group, respectively (Figure S3). In categorical analyses, adherence was defined as all or all but one of prescribed drugs detected in urine whereas nonadherence was two or more of drugs undetected. At baseline, nonadherence was observed in 4 (44%) patients randomized to control and 3 (50%) allocated to RDN. During follow-up, in the control group, nonadherence occurred in 2 patients (22%) at one visit, in 1 (11%) at two visits and in 3 (33%) at all visits. These numbers in the intervention group were 0 (0%), 2 (33%) and 2 (33%), respectively. Nonadherence at any time from baseline to the 6-month visit occurred in 8 (88.9%) patients vs. 4 (66.7%) randomized to control or RDN, respectively (P = .53). Figure S4 describes the change in adherence from baseline to the 6-month visit.
Quality of life
At baseline (Table S1), 44% of patients in the control group reported problems (from slight to extreme) related to mobility vs. 67% in the RDN group. Corresponding numbers for self-care, usual activity, pain or discomfort and anxiety or depression were 22%, 67%, 78% and 67% in the control group vs. 17%, 83%, 83%, 50% in the intervention group. At 6 months of follow-up 44%, 22%, 44%, 89% and 54% of patients in the control group reported to have problems related to mobility, self-care, usual activities, pain or discomfort and anxiety or depression, while in the RDN group these proportions were 50%, 17%, 67%, 84% and 50%, respectively. There were no between-group differences (P ≥ .14). At baseline, the self-reported health status according to the visual analogue scale was 53.9 ± 28.5 in the control group and 64.2 ± 21.5 in the intervention group and 53.8 ± 22.3 and 75.0 ± 14.1, respectively, at 6 months. The baseline-adjusted between-group difference in self-reported health status at 6 months was not significant: 13.6 (CI, −7.4 to 34.6; P = .28).
Discussion
The INSPiRED pilot study included a group of highly-selected patients with truly resistant hypertension. Six months after randomization, the 24-h and nighttime ambulatory blood pressure decreased significantly more in response to RDN on top of optimized antihypertensive drug treatment compared with sole optimization of drug treatment. The strong decrease in nighttime blood pressure can probably be explained by the more standardized conditions in terms of body position and absence of physical activity, when blood pressure was recorded during sleep [Citation27]. Office and daytime ambulatory blood pressure showed similar trends but did not reach significance (Figure S2). Decreases in the ECG voltage and voltage × QRS duration products and tapering of antihypertensive drugs in the RDN group provided additional evidence supporting a true blood pressure decrease in the intervention group. Drug adherence and quality of life were similar in both groups. Changes in serum creatinine and eGFR and quality of life were small and also similar in the two treatment groups. Finally, changes in blood pressure and adherence were unrelated. We therefore aim to engage in a definite trial, using the same protocol [Citation8].
In a review [Citation28] published 2 years before SYMPLICITY HTN-3 [Citation4], we highlighted several issues hampering the interpretation of studies of RDN available at that time. Our concerns included the uncontrolled design of several single-arm studies without randomization, the loose definition of resistant hypertension, the assurance that patients with secondary hypertension were excluded from enrollment, the use of office rather than ambulatory blood pressure as primary efficacy endpoint, the assessment of adherence to antihypertensive drug treatment as confounder and the completeness of RDN with the single-electrode SYMPLICITY™ RDN system [Citation28]. In a subject-level meta-analysis of 109 patients undergoing RDN, higher baseline serum creatinine predicted lower probability of improvement of the 24-h blood pressure (odds ratio for 20-mmol/L [0.18 mg/dL] increase, 0.60; P = .05) and higher probability of experiencing no blood pressure decrease (odds ratio, 1.66; P = .01) [Citation6].
Moreover, we highlighted that essential hypertension is a multifaceted disease, isolated systolic hypertension being characterized by arterial stiffening rather than heightened sympathetic tone and diastolic hypertension by an increased peripheral resistance in the long-term maintained by remodeling of small arteries [Citation28]. We concluded that end-stage hypertension in the presence of substantial target organ damage was unlikely to be responsive to RDN and that against the prevailing view at that time one size could not fit all [Citation28].
The aforementioned concerns [Citation5,Citation28] informed the design of INSPiRED, which sets this trial apart from most other studies of RDN in treatment-resistant hypertension. Specific design features include: (i) stringent patient selection, excluding patients with eGFR less than 45 ml/min/1.73 m2 or a body mass index of 40 kg/m2 or more, ineligibility of patients with isolated systolic or isolated diastolic hypertension or masked hypertension; (ii) age limited from 20 to 69 years inclusive; (iii) optimization of drug treatment according to a standardized strategy [Citation8,Citation10] with assessment of adherence throughout the study by means of the Morisky questionnaire [Citation22,Citation23] and urine analysis to ascertain the presence of antihypertensive drugs [Citation24,Citation25]; (iv) use of variable single-pill combinations that enhance adherence [Citation19] and long-acting forgiving [Citation18] drugs; (v) the explicit use of 24-h ambulatory blood pressure monitoring for selection of truly resistant patients and for assessment of the primary efficacy endpoint; (vi) thorough assessment of safety not only based on eGFR, but on renal imaging and detailed logging of the RDN procedure as well; (vi) and the use of a multi-electrode basket shaped RDN catheter, which compared with the SYMPLICITY™ single-electrode system allows delivering in a more precise manner radiofrequency energy simultaneously to four sites of an artery with a caliber suitable to be engaged. In addition, the leading interventional cardiologist (J.R.), who shared his experience across the three centers, was highly skilled based on his experience in SYMPLICITY HTN-2 [Citation3] and other RDN trials using different ablation systems.
In our current study, we ensured blinded endpoint assessment by having office blood and the 24-h blood pressure measured by automated devices, by using ECG measurements digitally exported from the recordings and by keeping investigators blinded to the results of the urinary assessment of drug adherence until completion of the pilot run. SAS programming guaranteed uniform extraction of the analyzed blood pressure and ECG outcomes. To our knowledge, our current report is the first to include a quality assessment of office and 24-h ambulatory blood pressure measurement. Furthermore, we recently completed a meta-analysis comparing the blood pressure responses to RDN vs. control in sham and non-sham controlled trials [Citation29]. In three sham controlled studies [Citation4,Citation30,Citation31] (number of patients randomized to intervention vs. control, 396 vs. 229) the decrease in the 24-h systolic blood pressure averaged 2.18 mm Hg (CI, −4.70 to 0.33 mm Hg; P = .09). For the six trials [Citation3,Citation32–36] with open design or other types of masking (162 vs. 174), this estimate was similar (P = .47) amounting to +0.42 mm Hg (CI, −6.20 to 7.46 mm Hg; P = .90). Abstraction made of these observations [Citation29], a sham-controlled trial is less likely to pass ethical review in Europe.
As already reported close to 30 years ago [Citation37] and confirmed since then in numerous studies [Citation19,Citation25], drug adherence is a major problem in patients with resistant hypertension. In line with previous publications [Citation19,Citation24,Citation38], adherence as assessed by urine analysis revealed that about 80% of the INSPiRED patients were nonadherent at some time during the randomized part of the pilot trial. The Morisky questionnaire has a much lower sensitivity to detect nonadherence [Citation39]. As far as we know, only three earlier RDN trials [Citation40–42] applied a stringent approach to assess adherence. In the Oslo trial (NCT01673516) [Citation40], 19 of 65 screened patients (29.2%) were excluded from randomization, because ambulatory blood pressure normalized after witnessed drug intake just before the qualifying visit. The Renal Denervation for Hypertension Trial (DENERHTN; NCT01570777) [Citation41] implemented a highly standardized treatment schedule. Drug adherence was assessed at the 6-month visit in 85 of 106 randomized patients (80.2%) by determining the urinary N-acetyl-seryl-aspartyl-lysyl-proline/creatinine ratio [Citation43] and by ultra-high performance liquid chromatography tandem mass spectrometry to detect the drugs in urine or plasma [Citation24]. The prevalence of nonadherence in DENERHTN was comparable in both treatment groups, amounting to approximately 50%, and as in the current study did not influence the systolic blood pressure gradient in favor of RDN relative to control [Citation41]. The Renal Sympathetic Denervation as a New Treatment for Therapy Resistant Hypertension Trial (SYMPATHY; NCT01850901) [Citation42] is the only RDN trial, in which as in our current study adherence was assessed by measuring drug concentrations both at baseline and follow-up. In 78 patients blood samples were drawn synchronously with blood pressure measurements. Neither patients nor physicians knew that adherence was being monitored. In 80% of patients, fewer medications were detected than prescribed and adherence changed during follow-up in 31% [Citation42].
Sailing against the tide after publication of SYMPLICITY HTN-3 [Citation4], and the overall null results of the trials with the SYMPLICITY™ mono-electrode RDN system [Citation5,Citation29] explains why we needed 26 months to randomize only 15 patients. First, referrals of patients for RDN by primary care physicians and cardiologists sharply declined after publication of SYMPLICITY HTN-3 [Citation4]. Second, most manufacturers of RDN systems lost interest, once it became obvious that a highly profitable and potentially huge market was in fact limited to a group of highly selected patients, in whom antihypertensive drug treatment was optimized in a standardized manner. St. Jude Medical (now Abbott [http://www.abbott.com/newsroom/news/abbotts-next-big-step.html]) stopped production of the EnligHTN™ multi-electrode system in July 2016, so that INSPiRED investigators were faced with the ethical dilemma that randomized patients could not undergo the procedure as part of the pilot trial or that control patients could not cross over at 6 months. Until today, production of the EnligHTN™ cathethers has not yet been resumed. Third, while submitting INSPiRED to the ethical review boards of the three participating tertiary referral centers, we were only granted to start a pilot trial with the obligation to report back after up to 18 patients had been randomized.
Set aside from its design features, the current study must be interpreted within the context of its limitations. Obviously, the number of patients randomized and analyzed, in keeping with ethical recommendations, was small and two patients randomized to RDN did not undergo the procedure. Although the blood pressure results were supported by lower ECG voltages and fewer prescribed drugs in the RDN group, a pilot trial cannot answer research objectives beyond doubt. Second, our trial does not allow comparing RDN systems (see online-only Supplementary Data page 8). Finally, our trial did not yet implement an immediate procedural endpoint. As we reported before, renal nerve stimulation cannot only identify areas in the renal arteries that should be preferentially denervated [Citation44], but also predict the blood pressure response to RDN [Citation45].
In view of the positive outcome of the INSPIRED pilot trial, the ENCOReD consortium will seek ethical approval for implementation of the study protocol across multiple hypertension centers not only in Belgium, but across Europe as well, without change of the protocol [Citation8]. A substudy will address the role of renal nerve stimulation in identifying the anatomical sites in the renal arteries to be denervated and as a means to obtain an immediate procedural endpoint [Citation44,Citation45]. Patients in this substudy will be randomized to guided vs. unguided RDN. Sample size, originally estimated to amount to 120 per group to address the primary (2-sided α, 0.01; β, 0.90) and secondary (2-sided α, 0.05; β, 0.95) endpoints [Citation8], will not be reduced in spite of the positive outcome of the pilot study, but an interim analysis will be performed for the primary and secondary endpoints after 60 patients in each group have completed the 6-month visit. If different RDN systems will be used, device type will constitute an additional level of stratification before randomization, allowing – power permitting – a predefined subgroup analysis comparing devices.
Isplt5_spl_jas.pdf
Download PDF (372.1 KB)Acknowledgements
The authors gratefully acknowledge the expert clerical assistance of Mrs. Vera De Leebeeck and Mrs. Renilde Wolfs (Studies Coordinating Centre, Leuven, Belgium). INSPiRED is conducted under the auspices of the Belgian Hypertension Committee (http://www.belhyp.be). The authors are grateful to Jean-François De Wispelaere, Charles Cuvelier and Jean-Michel Pochet (CHU UCL Dinant Godinne) and Michel Henry, Albertino Maimone, Abdelhamid Lalaoui, and Philippe Leroy (CHR Mons Hainaut, Mons) for their contribution to patient screening and referral and to Mrs Francesca Severino and Amandine Jourdan, research nurses, for coordinating the study at the Cliniques Universitaires Saint-Luc (UCL) and the Grand Hôpital de Charleroi, respectively.
Disclosure statement
INSPiRED is an investigator driven trial not supported by any manufacturer of renal denervation systems. None of the authors declares a conflict of interest.
Additional information
Funding
References
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117:e510–e526.
- Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281.
- Symplicity HTN-2 Investigators, Esler MD, Krum H, Sobotka PA, et al. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903–1909.
- Bhatt DL, Kandzari DE, O'Neill WW, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–1401.
- Fadl Elmula FE, Jin Y, Yang WY, et al. Meta-analysis of randomized controlled trials of renal denervation in treatment-resistant hypertension. Blood Press. 2015;24:263–274.
- Persu A, Jin Y, Azizi M, et al. Blood pressure changes after renal denervation at 10 European expert centers. J Hum Hypertens. 2014;28:150–156.
- Persu A, Azizi M, Jin Y, et al. Hyperresponders vs. nonresponder patients after renal denervation: do they differ? J Hypertens. 2014;32:2422–2427.
- Jin Y, Jacobs L, Baelen M, et al. Rationale and design of the Investigator-Steered Project on Intravascular Renal Denervation for Management of Drug-Resistant Hypertension (INSPiRED) trial. Blood Press. 2014;23:138–146.
- World Medical Association. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. J Am Med Ass. 2013;310:2191–2194.
- British Cardiac Society, British Hypertension Society, Diabetes UK, HEART UK, Primary Care Cardiovascular Society, The Stroke Association. JBS 2: Joint British Societies’ guidelines on prevention of cardiovascular diseases in clinical practice. Heart. 2005;91:1–52.
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612.
- O'Brien E, Asmar R, Beilin L, et al. Practice guidelines of the European Society of Hypertension for clinic, ambulatory and self blood pressure measurement. J Hypertens. 2005;23:697–701.
- O'Brien E, Parati G, Stergiou G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731–1768.
- O'Brien E, Mee F, Atkins N, et al. Evaluation of three devices for self-measurement of blood pressure according to the revised British Hypertension Society Protocol: the Omron HEM-705 CP, Philips HP5332 and Nissei DS-175. Blood Press Monit. 1996;1:55–62.
- Franssen PML, Imholz BPM. Evaluation of the Mobil-O-Graph new generation ABPM device using the ESH criteria. Blood Press Monit. 2010;15:229–231.
- Barna I, Keszei A, Dunai A. Evaluation of the Meditech ABPM-04 ambulatory blood pressure measuring device according to the British Hypertension Society protocol. Blood Press Monit. 1998;3:363–368.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–2219.
- Hernández-Hernández R, Armas de Hernández MJ, Armas-Padilla MC, et al. The effect of missing dose of enalapril versus amlodipine on ambulatory blood pressure. Blood Press Monit. 1996;1:121–126.
- Burnier M, Wuerzner G, Struijker-Boudier H, et al. Measuring, analyzing, and managing drug adherence in resistant hypertension. Hypertension. 2013;62:218–225.
- Liu PS, Platt JF. CT angiography of the renal circulation. Radiol Clin North Am. 2010;48:347–365.
- Worthley SG, Tsioufis CP, Worthley MI, et al. Safety and efficacy of a multi-electrode renal sympathetic denervation system in resistant hypertension: the EnligHTN I trial. Eur Heart J. 2013;34:2132–2140.
- Morisky DE, Ang A, Krousel-Wood M, et al. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich). 2008;10:348–354.
- Korb-Savoldelli V, Gillaizeau F, Pouchot J, et al. Validation of a French version of the 8-item Morisky medication adherence scale in hypertensive adults. J Clin Hypertens (Greenwich). 2012;14:429–434.
- Jung O, Gechter JL, Wunder C, et al. Resistant hypertension? Assessment of adherence by toxicilogical urine analysis. J Hypertens. 2013;31:766–774.
- Schmieder RE, Ott C, Schmid A, et al. Adherence to antihypertensive medication in treatment-resistant hypertension undergoing renal denervation. J Am Heart Assoc. 2016;5:e002343.
- EuroQol Group. EuroQol: a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208.
- Staessen JA, Li Y, Hara A, et al. Blood pressure measurement Anno 2016. Am J Hypertens. 2017;30:453–463.
- Persu A, Renkin J, Thijs L, et al. Renal denervation: ultima ratio or standard in treatment-resistant hypertension. Hypertension. 2012;60:596–606.
- Fadl Elmula FE, Feng Y, Jacobs L, et al. Sham or no sham control: that is the question in trials of renal denervation for resistant hypertension. A systematic meta-analysis. Blood Press. 2017 [cited 2017 Apr 26]. DOI: 10.1080/08037051.2017.1311769
- Desch S, Okon T, Heinemann D, et al. Randomized sham-controlled trial of renal sympathetic denervation in mild resistant hypertension. Hypertension. 2015;62:1202–1208.
- Mathiassen ON, Vase H, Bech JN, et al. Renal denervation in treatment-resistant essential hypertension. A randomized, SHAM-controlled, double-blinded trial 24-h blood pressure-based trial. J Hypertens. 2016;34:1639–1647.
- Fadl Elmula FE, Hoffmann P, Larstorp AC, et al. Adjusted drug treatment is superior to renal sympathetic denervation in patients with true treatment-resistant hypertension. Hypertension. 2014;63:691–699.
- Rosa J, Widimský P, Toušek P, et al. Randomized comparison of renal denervation versus intensified pharmacotherapy including spironolactone in true-resistant hypertension: six-month results from the Prague-15 study. Hypertension. 2014;65:407–413.
- Azizi M, Sapoval M, Gosse P, et al. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet. 2015;385:1957–1965.
- Kario K, Ogawa H, Okumura K, et al. SYMPLICITY HTN-Japan: first randomized controlled trial of catheter-based renal denervation in Asian patients. Circ J. 2015;79:1222–1229.
- Oliveras A, Armario P, Clarà A, et al. Spironolactone versus sympathetic renal denervation to treat true resistant hypertension: results from the DENERVHTA study: a randomized controlled trial. J Hypertens. 2017;34:1863–1871.
- Gifford RW. An algorithm for the management of resistant hypertension. Hypertension. 1988;11:171–175.
- Berra E, Azizi M, Capron A, et al. Evaluation of adherence should become an integral part of assessment of patients with apparently treatment-resistant hypertension. Hypertension. 2016;68:297–306.
- Pandey A, Raza F, Velasco A, et al. Comparison of Morisky Medication Adherence Scale with therapeutic drug monitoring in apparent treatment resistant hypertension. J Am Soc Hypertens. 2015;9:420–426.
- Fadl Elmula FE, Hoffmann P, Fossum E, et al. Renal sympathetic denervation in patients with treatment-resitant hypertension after witnessed intake of medication before qualifying ambulatory blood pressure. Hypertension. 2013;62:526–532.
- Azizi M, Pereira H, Hamdidouche I, et al. Adherence to antihypertensive treatment and the blood pressure lowering effects of renal denervation in the Renal Denervation for Hypertension (DENERHTN) Trial. Circulation. 2016;134:847–857.
- de Jager RL, de Beus E, Beeftink MMA, et al. The impact of medication adherence on the effect of renal denervation. The SYMPATHY trial. Hypertension. 2017;69:678–684.
- Azizi M, Ménard J, Peyrard S, et al. Assessment of patients’ and physicians’ compliance to an ACE inhibitor treatment based on urinary N-Acetyl Ser-Asp-Lys-Pro determination in the Noninsulin-Dependent Diabetes, Hypertension, Microalbuminuria, Proteinuria, Cardiovascular Events and Ramipril (DIABHYCAR) study. Diabet Care. 2006;29:1331–1336.
- Gal P, de Jong MR, Smit JJ, et al. Blood pressure response to renal nerve stimulation in patients undergoing renal denervation: a feasibility study. J Hum Hypertens. 2015;29:292–295.
- de Jong MR, Adiyaman A, Gal P, et al. Renal nerve stimulation-induced blood pressure changes predict ambulatory blood pressure response after renal denervation. Hypertension. 2016;68:707–714.
