Abstract
Background: The use of automated (oscillometric) blood pressure (BP) devices is not validated in atrial fibrillation (AF) patients.
Objectives: To assess the reliability of three oscillometric BP devices, and the agreement with invasive arterial blood pressure(IBP) in AF patients.
Methods: 48 AF patients with randomized sequences of 10 consecutive BP measurements with two pairs of devices: (1) OmronR7™(wrist) and OmronHEM907™(arm); (2) OmronR7™ and Microlife WatchBPhome(arm). Reliability and agreement of each device were assessed by the intra-class correlation coefficient (ICC) for the continuous BP measurements and Bland & Altman methodology, respectively. In 10 additional AF patients, 10 consecutive measurements with IBP and OmronHEM907™, and IBP and Microlife WatchBPhome were performed.
Results: The OmronR7™ was not able to obtain any BP Readings. Arm devices presented better ICC for systolicBP(SBP) than for diastolicBP(DBP) (Omron HEM907™:0.94 [0.90; 0.97] vs. 0.77 [0.67; 0.89]; Microlife WatchBPhome:0.92 [0.88; 0.96] vs.0.79 [0.69; 0.89]).The correlation coefficient between Microlife WatchBPhome and IBP computed using the average of repeated measurements from two to ten measurements improved up to the third and remained stable afterwards.
The agreement between IBP and SBP, and IBP and DBP, was moderate as illustrated by a wide limit of agreement [−24; 26](SBP) and [−15;17](DBP) for Microlife WatchBPHome, respectively and [−30; 13](SBP) and [−7; 15](DBP) for OmronHEM907.
Conclusions: BP measurement using the two arm oscillometric devices achieved a high reliability for SBP. The agreement between IBP and arm devices was low but using the average of three consecutive measurements improved the results substantially.
Introduction
Blood pressure (BP) measurement is one of the most common medical procedures. It is used in a wide variety of clinical settings from diagnosis of hypertension in the ambulatory care to monitoring of BP in intensive care units. The joint national committee (JNC) 7 and the European Hypertension Society recommend the use of a calibrated mercury sphygmomanometer to measure office BP in patients with sinus rhythm, but the use of an oscillometric device is also considered as valid if a mercury sphygmomanometer is not available. In presence of atrial fibrillation (AF), the use of a manual device (auscultatory method) is recommended, whereas an oscillometric device can be used for self BP monitoring [Citation1–3]. However, mercury sphygmomanometers tend to be less employed, due to environmental concerns and their relative cumbersome use compared to oscillometric devices [Citation4]. In consequence, automatic oscillometric devices are increasingly used to measure BP in hospitals even in patients with AF. Nonetheless, most of those devices have not been properly validated in such patients [Citation5]. Mainly because in atrial fibrillation, there is uncertainty (BP variability, between observers' agreement) with the reference method for BP measurement in AF which limits the used of standard validation protocols such as the ISO or ESH protocols which exist for device validation outside the AF setting [Citation6,Citation7].
A recent meta-analysis by Stergiou et al. showed that among patients with AF, the evidence coming from validation studies of automated devices was limited and that study results were heterogeneous [Citation8].
Given the aging of the general population, the prevalence of both AF and hypertension is increasing [Citation9]. Accurate measurement of BP using easy to use devices is therefore important for diagnostic and therapeutic purposes in patients with AF.
The objectives of this study were to validate automated BP devices in patients with AF by assessing: (1) the reliability of three devices (two arm, and one wrist, commonly used oscillometric devices); (2) their precision; (3) their accuracy; (4) and the agreement of each device with intra-arterial radial BP measurements in a hospital setting.
Study design
Ethics
The trial was conducted after the approval of the Ethical commission of Canton Vaud (www.cer-vd.ch). All participants provided written informed consent.
Devices
Three commonly used BP measuring devices (two arms, and one wrist) were used: the Omron HEM907TM (OHEM) (a professional office BP monitor device) is the arm device used in our hospital for BP monitoring in ambulatory and hospitalized patients and two devices developed for self-home BP monitoring by patients: the Microlife WatchBPHomeTM (MWBP) another arm device with an algorithm designed to detect AF [Citation9] and, the Omron R7TM (OR7) a wrist device. All three oscillometric devices were previously validated for subjects in sinusal rhythm using standard protocols [Citation10–12].
Participants
Eligible subjects were recruited during their hospitalization in the Department of Internal Medicine of Lausanne University Hospital. A group of 10 additional patients with invasive BP monitoring through an arterial indwelling catheter was recruited in the Intensive Care Unit of the same hospital. This group was used as a reference standard to investigate the accuracy and the agreement of the oscillometric devices. All subjects had to present with AF at the time of the measurement to be included in the final analysis.
Exclusion criteria were the presence of a pacemaker, of an arterio-venous fistula, signs of infection at the site of BP measurement and age <18 years old.
Measurements
Blood pressure was measured by a single research nurse trained in the use of the devices, following the recommendation of the JNC 7 [Citation3].
All patients were instructed not to drink coffee and to refrain from smoking or exercising 30 minutes before measurements. Depending on patient disability, BP was measured in a sitting or in a semi-recumbent position. For the arm devices, the length of the inflatable bladder of the cuff (medium: between 22 to 32 or large: 32 to 42 cm) was selected according to arm circumference. The bladder should cover at least over 80% of the arm circumference which corresponded for all the patients as to using the cuff size recommended by the manufacturer.
The cuff was placed 2 cm above the left elbow. The wrist device was placed around the left wrist at the same level as the cuff from the arm device (“heart level”). To reproduce the “real conditions” of hospital setting, BP was measured in the patient’s hospital room.
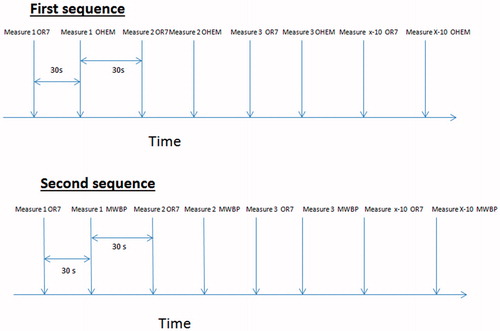
For each participant, two distinct pairs of BP devices (OR7 + OHEM and OR7 + MWBP) were consecutively used in two separate sequences (i.e. one for each pair of device) consisting in 10 BP measurements. The two sequences were separated by a 5 minutes pause, whereas, within a sequence, measurements were separated by a 30-second lag period. Both the sequences (i.e. which pair of BP devices was to be used first) and order of use of devices within a pair were randomized.
More precisely, wrist and arm devices randomized to be used first (i.e. corresponding to the first pair) were positioned on the left arm for all participants. Then, another randomization process determined which of the two devices was the first to be used, the first device being called “device A”, the second “device B”. Each device was used 30 seconds apart. Concretely, a first measurement was taken with “device A”, which was followed 30 seconds later with a measurement with “device B”. Then another measurement was taken with “device A” and a second measurement was taken with “device B” .This sequence was repeated up to 10 measurements for each device. At the end of the first sequence, the cuff of the arm device was removed and replaced by the cuff of the arm device from the second pair. After a pause of 5 minutes, the second sequence was undertaken as described for the first pair of devices ().
Figure 1. Example of a measuring sequence. In this example, the first sequence is OmronR7-OmronHEM907TM, the second is OmronR7- Microlife WatchBPHome™. In this sequence, the OmronR7 is the first device in both sequences (“device A”), both arm devices are “device B” in this example.
The same procedure was applied in the group of patients with an intra-arterial catheter except that the pairs of devices were: (1) OHEM + intra-arterial blood pressure (IBP); and (2) MWBP + IBP. For the IBP, the values of the BP displayed on the patient intensive care monitor were recorded when the oscillometric device indicated a BP value. For all IBP measurements, the zero reference level was set at the level of the right atrium (phlebostatic point).
If any device failed to measure BP twice consecutively, the procedure was stopped. A three lead electrocardiogram recorded the cardiac rhythm of the patients during the procedure. If the average values of BP measurements with a device were >140 mm Hg for systolic BP (SBP) or >90 mm Hg for diastolic BP (DBP), the patient was defined as hypertensive with this device.
Data
Clinical (hypertension, diabetes, renal insufficiency, use of anti-hypertensive drugs and circumference of the arm) and demographic (sex, age) data were collected using the patient’s medical file.
Statistical analysis
Statistical analyses were conducted using Stata version 13.1 for Windows® (Stata corp, College Station, TX, USA). Results were expressed as mean ± standard deviation for continuous variables and as number of patients (percentage) for categorical variables.
The reliability of devices to consistently provide similar results was assessed by the intra-class correlation coefficient and their precision by the within subject standard deviation [Citation13,Citation14].
Agreement between each device and IBP measurements was investigated by the Bland & Altman limits of agreement (LoA) method and accuracy quantified by the mean bias [Citation15].
Reliability of devices and IBP measurements regarding the diagnosis of hypertension (SBP >140 mm Hg or DBP >90 mm Hg) was assessed by the kappa statistic: (1) using all individual measurements and (2) using the mean value of the repeated measurements for each participant .Finally, the percentage of absolute difference between BP in the 5, 10 and 15 mm Hg range between IBP and MWBP was computed. Due to the limited number of measurements between OHEM and IBP, this was not computed between those devices. To compare our data with the AAMI protocol, we have computed mean difference between the test device and the standard (i.e. the oscillometric devices and IBP) [Citation7].
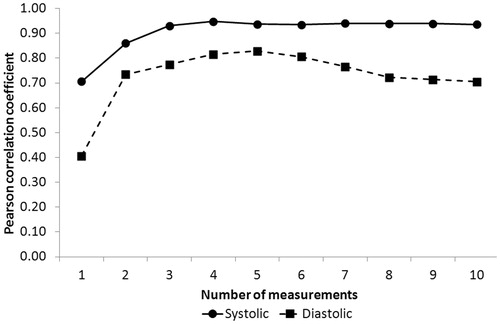
To illustrate that taking the mean of the individual repeated measurements yields a clinically significant improvement of the correlation between device and IBP, the Pearson correlation coefficient between IBP and MWBP was computed by using the individual mean BP values (instead of the repeated measurements). We did this consecutively by using the first 2, 3, 4, etc., repeated measurements in each individual.
Surmising that BP varies with each cardiac beat in patients with AF, we hypothesized that BP in a given period of time was best estimated by the mean BP derived from all measurements performed during that period. Thus, the analysis based on the mean BP of each subject is called “mean BP” thereafter.
Results
Patients’ recruitment and characteristics
From October 2013 to August 2015, 95 patients were screened in the Department of Internal Medicine; ten refused to participate, 33 left the hospital before the measurements. Three withdrew their consent, one was excluded because he was not in AF at the time of measurement and 48 patients were included in the final analysis (Supplemental Figure A). In parallel, 21 patients with an intra-arterial catheter were screened in the Intensive Care Unit: 12 were recruited and 10 were included in the final analysis (Supplemental Figure B).
The clinical and demographic data of the participants are shown in . Their mean (±SD) age was 80 (±10) years and 70.8% of them had a diagnosis of hypertension at the beginning of the study. Two thirds (65%) were treated with a β-blocker (No Intensive Care group) (Supplemental Table A). The average pulse rate was 96 ± 14 bpm for the ICU group.
Table 1. Characteristics of the sample.
Devices’ reliability and precision
The wrist device, OR7, was unable to provide any measurement in all the participants. On the other hand, the MWBP provided ten measures consecutively in 87% of the patients (with a mean number of 9.3 ± 2.3 measurements/subject) and the OHEM 5.3 ± 4.5 measurements/subject (WPB vs. OHEM p < .001) ().
Table 2. Performance of arm devices.
The ICC (i.e. reliability) for SBP measured by the MWBP device was 0.92 (95%CI [0.88; 0.96]) and 0.79 (95%CI [0.69; 0.89]) for DBP. The within-subject standard deviation (i.e. precision) was 4.8 mm Hg for SBP and 4.2 for DBP. For the OHEM device, ICC was 0.94 (95% confidence interval [0.90; 0.97]) for SBP and 0.77 (95% confidence interval [0.67; 0.89]) for DBP. The within-subject standard deviation was 4.6 mm Hg and 4.8 mm Hg for OHEM SBP and DBP, respectively ().
Table 3. Intraclass correlations according to device (arm measurements).
As for IBP the ICC was 0.90 (95% confidence interval [0.82; 0.99]) for the SBP and 0.84 (95% confidence interval [0.71; 0.97]) for the DBP respectively. The within-subject standard deviations were 6.2 mm Hg and 5.3 mm Hg for SBP and DBP, respectively.
Finally, the reliability of the two devices regarding the diagnosis of hypertension was moderate (Kappa =0.59, 95%CI [0.20; 0.83] (Supplemental Table B). The reliability of the devices in regard to IBP regarding the categorization of a patient as hypertensive or normotensive was better for OHEM than for MWBP (Kappa values of 1.00 and 0.69, respectively). The reliability improved when the mean BP values were used ().
Table 4. Reliability regarding the diagnosis of hypertension between intra-arterial and peripheral blood pressure measurement.
Due to the inability of the OR7 to measure any BP, no statistical analysis was performed with this device.
Devices’ accuracy and agreement (in relation to IBP)
The MWBP provided more BP measurements than the OHEM (96 vs. 57) because there were less failure to report readings with the MWBP. The accuracy of the MWBP device was good with a mean bias of 1.0 mm Hg for SBP and 9.4 mm Hg for DBP. For the OHEM device, the means bias was -8.3 mm Hg for SBP and 4.2 mm Hg for DBP.
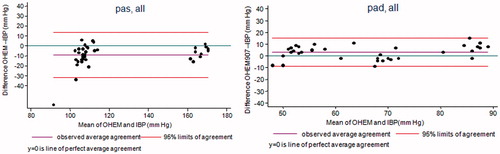
The agreement figures between the MWBP and IBP, and between the OHEM and IBP, are provided in and . When individual repeated measures were used, the LoA for the MWBP were [−24; 26] mm Hg for SBP and [−18; 37] mm Hg for DBP, and [−30; 13] mm Hg for SBP and [−7; 15] mm Hg for DBP for the OHEM. When the individual means of the BP values were used instead, the LoA were narrower: [−15; 17] mm Hg for SBP and [−10; 29] mm Hg for DBP for the MWBP and [−30; 9] mm Hg for SBP and [−12; 6] mm Hg for DBP for the OHEM.
Figure 2. Bland & Altman plot between Microlife WatchBPhome™ and Invasive Blood Pressure. The Microlife WatchBPhome™ systematically overestimated systolic and diastolic blood pressure.
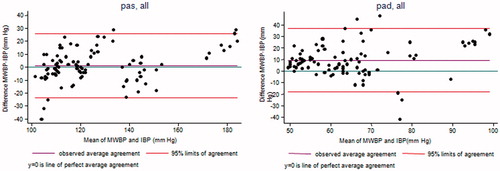
Figure 3. Bland & Altman plot between OmronHEM907™ and Invasive Blood Pressure. The OmronHEM907™ systematically underestimated systolic and diastolic blood pressure.
The percentage of absolute difference between BP in the 5, 10 and 15 mm Hg range between IBP and MWBP were 41% of in a 5 mm Hg range, 63% in a 10 mm Hg range and 75% in a 15 mm Hg range.
The correlation coefficient between IBP and MWBP computed by using the individual mean BP values substantially improved between the first and third consecutive measurements but remained stable afterwards ().
Discussion
Devices’ reliability and precision
This study shows that the selected arm devices achieved a high SBP reliability in patients with AF. This was supported by the fact that all devices fulfilled the criteria of precision of the AAMI protocol [Citation6,Citation7]. Arm devices precision and accuracy improved when mean BP was used compared to individual BP measurements. Morevover the OHEM is more accurate for diastolic BP than for systolic BP and whereas the MWBP is more accurate for systolic BP than for diastolic BP. Finally, our findings confirmed that a wrist device is unsuitable to measure BP in patients in AF.
The inability for the wrist device (OR7) to provide any measurement at all makes its use inappropriate for BP measurement in patients with AF. This finding is consistent with previous observations where a wrist device (Omron R5™) underperformed in patients with AF [Citation16]. In the study of Pagonas et al, although the device was able to measure BP, it failed to fulfill the criteria of validation of the AAMI protocol [Citation17]. Therefore, our findings confirm that the use of wrist devices to measure BP in patients with AF is not warranted in clinical practice.
The MWBP, which has an algorithm specifically designed to detect AF, provided 10 consecutive BP measurements in almost 100% of our subjects. In comparison, the OHEM was only able to provide an average of 5 measurements out of the 10 attempts. Whether this detection rate has any relevant clinical implication is speculative, but could influence patients' confidence in the device. However, based on recommendations of hypertension societies, a BP device is declared suitable when it is able to consecutively measure BP thrice. Hence, both devices seem adequate in that respect [Citation2].
Both arm devices were reliable given the high ICC. The ICCs for SBP obtained in both devices were nearly the same as the ICC obtained with a mercury sphygmomanometer in patients without AF [Citation18]. The performance of the OHEM and the MWBP in patients in AF was then comparable to that in patients with sinusal rhythm, confirming optimal reliability of the devices for patient in AF [Citation19]. However, it is noteworthy to mention that the ICC for DBP was smaller than for SBP for both devices, suggesting a lower reliability for DBP measurements. Interestingly, the within-subject standard deviation of SBP and DBP measured by IBP (6.2 mm Hg for SBP and 5.3 mm Hg for DBP) was nearly the same as the one measured by the oscillometric devices (4.8 and 4.6 mm Hg for SBP and 4.2 and 4.8 mm Hg for DBP). This showed that the precision of the oscillometric devices was as good as IBP, which represents the gold standard. Interestingly, in the meta-analysis by Stergiou et al [Citation8], SBP measurements were better correlated between the oscillometric devices and mercury sphygmomanometers devices than DBP measurements. This highlight that correlation is not a good surrogate for precision, and should not be used to compare different devices for BP measurement, since it cannot exclude a systematic error. Concerning the diagnosis of hypertension, the reliability between devices based on the Kappa was rated as fair using the definition by Fleiss [Citation20]. Indeed Graves et al used a Kappa value of 0.8 to conclude that the BpTRUTM was as reliable as a trained nursed with a mercury sphygmomanometer to diagnostic hypertension [Citation21–23].
Devices’ accuracy and agreement
To further assess the agreement of the devices, we computed for each one the LoA using the IBP as the reference. We found presence of wide range of LoA that suggested a moderate agreement between devices and IBP. It is known that when compared to oscillometric devices, IBP tends to provided higher systolic BP and lower diastolic BP [Citation24]. This was the case with the OHEM systolic and diastolic BP and with the MWBP diastolic BP. However the MWPB systolic BP was higher than IBP, we believe this is due to the specific algorithm of this device.
However, when comparing the MWBP with IBP, the absolute mean difference for SBP satisfied the criteria of accuracy (< = 5 mm Hg difference) based on the AAMI protocol for validation of a BP device, but not for the DBP.
The criterion for precision (± 8 mm Hg SD) was fulfilled for all devices for both SBP and DBP. It is noteworthy to mention that in the formal AAMI protocol a mercury sphygmomanometer and not invasive BP is used as the gold standard.
In a study comparing BP measured with a mercury sphygmomanometer to intra-arterial BP in patients with sinus rhythm, the authors concluded that there was a good agreement between the two methods [Citation25,Citation26]. However, we are not aware of studies comparing mercury sphygmomanometer to invasive BP in patients with AF. Due to the great variability in cardiac beats in patients with AF, it is expected to find variations between manual monitoring and invasive BP. Since we could not exclude such bias, we decided to determine oscillometric BP devices’ accuracy and reliability compared to invasive measurements that represent the best approach to determine BP. From a clinical perspective, as the reliability based on the Kappa between the devices and IBP to diagnose hypertension was good for both devices, both arm devices could be proposed as a diagnostic tool for hypertension in patients with AF [Citation23].
Nonetheless, when using the individual mean BP values instead of the repeated measurements reduced variability between measures substantially, LoA improved greatly as well, as reliability to define a patient as being hypertensive. This procedure is in line with the recommendations from the AHA that propose to take several measurements of BP and to average them [Citation27]. Although our mean BP was based on 10 measurements (a scenario that is difficult to achieve in clinical practice), we showed that improvement in correlation was negligible when the BP was computed on more than three measurements. These findings thus support the measurement of BP using an oscillometric arm device in patients with AF. Consistent with recommendations from societies of hypertension, a reliable BP value should be obtained by averaging the values of at least three measures of BP [Citation21].
Conclusion
The measurement of BP in patients with AF is challenging and oscillometric devices are still less reliable in presence of AF. However, the automated arm devices, namely the Microlife WatchBPhome and the Omron HEM907TM, are valid for use in clinical practice when accounting for their biases. In line with previous reports and endorsing the recommendations of the AHA, the mean BP, derived from at least three measurements, should be used to obtain reliable and accurate values.
Limitation
Our study has some limitations that must be accounted for. First, our devices were not compared to ausculatory measurements. It is true that ausculatory method is the method used to validate an oscillometric device, however it as several bias and is not the true gold standard of blood pressure monitoring. Moreover our study was not designed to validate oscillometric devices. The purpose of our study was to measure several key surrogates of efficacity of oscillometric devices as in a daily hospital practice. Thus, by comparing our devices against invasive blood pressure and not the auscultatory method, we were able to test them against the true gold standard. Secondly, our study population was mainly composed of elderly hospitalized polymorbid patients, which can compromise the external validity of our findings to other settings. Thirdly, the majority of patients used sz-blockers and thus had a heart frequency below 100, which might decrease the validity of our findings in patients with AF and tachycardia. Finally in the ICU group, BP was taken with the oscillometric devices on the controlateral arm of the intra-arterial catheter.
Prior presentations
Preliminary results have been presented at the European congress of hypertension 2016 in Paris.
Supplemental_content.zip
Download Zip (64.4 KB)Acknowledgements
The study was integrally supported by the service of internal medicine of Lausanne University Hospital.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520.
- Parati G, Stergiou GS, Asmar R, et al. European Society of Hypertension guidelines for blood pressure monitoring at home: a summary report of the Second International Consensus Conference on Home Blood Pressure Monitoring. J Hypertens. 2008;26:1505–1526.
- Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252.
- Watson T, Lip GY. Blood pressure measurement in atrial fibrillation: goodbye mercury? J Hum Hypertens. 2006;20:638–640.
- Kollias A, Stergiou GS. Automated measurement of office, home and ambulatory blood pressure in atrial fibrillation. Clin Exp Pharmacol Physiol. 2014;41:9–15.
- Stergiou GS, Karpettas N, Atkins N, et al. European Society of Hypertension International Protocol for the validation of blood pressure monitors: a critical review of its application and rationale for revision. Blood Press Monit. 2010;15:39–48.
- AAMI. Electronic or automated sphygmomanometers. Association for the Advancement of Medical Instrumentation. 1993.
- Stergiou GS, Kollias A, Destounis A, et al. Automated blood pressure measurement in atrial fibrillation: a systematic review and meta-analysis. J Hypertens. 2012;30:2074–2082.
- Wiesel J, Abraham S, Messineo FC. Screening for asymptomatic atrial fibrillation while monitoring the blood pressure at home: trial of regular versus irregular pulse for prevention of stroke (TRIPPS 2.0). Am J Cardiol. 2013;111:1598–1601.
- Stergiou GS, Tzamouranis D, Protogerou A, et al. Validation of the Microlife Watch BP Office professional device for office blood pressure measurement according to the International protocol. Blood Press Monit. 2008;13:299–303.
- Topouchian JA, El Assaad MA, Orobinskaia LV, et al. Validation of two automatic devices for self-measurement of blood pressure according to the International Protocol of the European Society of Hypertension: the Omron M6 (HEM-7001-E) and the Omron R7 (HEM 637-IT). Blood Press Monit. 2006;11:165–171.
- Omboni S, Riva I, Giglio A, et al. Validation of the Omron M5-I, R5-I and HEM-907 automated blood pressure monitors in elderly individuals according to the International Protocol of the European Society of Hypertension. Blood Press Monit. 2007;12:233–242.
- Lin L. Overview of agreement statistics for medical devices. J Biopharm Stat. 2008;18:126–144.
- de Vet HC, Terwee CB, Knol DL, et al. When to use agreement versus reliability measures. J Clin Epidemiol. 2006;59:1033–1039.
- Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135–160.
- Pagonas N, Schmidt S, Eysel J, et al. Impact of atrial fibrillation on the accuracy of oscillometric blood pressure monitoring. Hypertension. 2013;62:579–584.
- Stergiou GS, Kollias A, Karpettas N. Does atrial fibrillation affect the automated oscillometric blood pressure measurement? Hypertension. 2013;62:e37.
- Alpert BS, Quinn DE, Friedman BA. A review of the latest guidelines for NIBP device validation. Blood Press Monit. 2013;18:297–302.
- Cao X, Song C, Guo L, et al. Quality control and validation of oscillometric blood pressure measurements taken during an epidemiological investigation. Medicine (Baltimore). 2015;94:e1475.
- Fleiss JL. Statistical methods for rates and proportions. 2nd ed. New York: John Wiley; 1891.
- Graves JW, Nash C, Burger K, et al. Clinical decision-making in hypertension using an automated (BpTRU) measurement device. J Hum Hypertens. 2003;17:823–827.
- Khan KS, Chien PF. Evaluation of a clinical test. I: assessment of reliability. BJOG. 2001;108:562–567.
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174.
- Picone DS, Schultz MG, Otahal P, et al. Accuracy of Cuff-Measured Blood Pressure: systematic reviews and meta-analyses. J Am Coll Cardiol. 2017;70:572–586.
- White WB, Lund-Johansen P, McCabe EJ, et al. Clinical evaluation of the Accutracker II ambulatory blood pressure monitor: assessment of performance in two countries and comparison with sphygmomanometry and intra-arterial blood pressure at rest and during exercise. J Hypertens. 1989;7:967–975.
- White WB, Lund-Johansen P, Omvik P. Assessment of four ambulatory blood pressure monitors and measurements by clinicians versus intraarterial blood pressure at rest and during exercise. Am J Cardiol. 1990;65:60–66.
- Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697–716.