Abstract
Purpose: Retinal microcirculation represents an easily accessible, non-invasive, in-vivo possibility to assess early microvascular changes. In addition to the assessment of functional (e.g. retinal capillary flow, RCF) and retinal arteriolar structural parameters (e.g. wall-to-lumen-ratio, WLR) we now suggest a new parameter reflecting the resistance in small retinal arterioles (RVR).
Material and methods: In 45 normotensive (NT) subjects and 123 patients with hypertension stage 1 (HT) we assessed RCF, WLR, arteriolar diameter, lumen diameter and wall cross section area in the retinal circulation by using scanning laser Doppler flowmetry (SLDF). Mean arterial pressure (MAP) was measured immediately before the SLDF measurement and retinal vascular resistance was calculated (RVR = MAP/RCF). In a separate study the test-retest reliability was determined in 6 volunteers from our clinical staff by assessing RVR three times within six weeks.
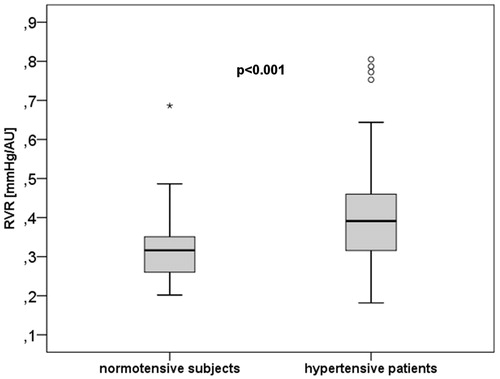
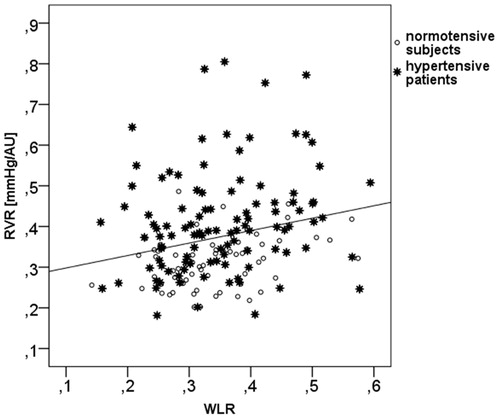
Results: The analysis of the volunteers revealed a coefficient of variation for RVR of 7.75 ± 2.11% and Cronbach´s alpha was 0.90. WLR, a marker of vascular remodeling did not differ between NT and HT. In contrast, RCF and inner diameter of the retinal arterioles (ID) were significantly lower (RCF: p = .045 and ID: p = .001) in the HT group than in the NT group and RVR was significantly higher in the HT group than in the NT group (p < .001). In both groups we found no correlation of RVR with age, but a significant correlation of RVR with WLR (NT: r = 0.34, p = .006; HT: r = 0.25, p = .01), indicating that the RVR reflects vascular remodeling in the retinal circulation.
Conclusion: Our data showed an increased retinal vascular resistance in hypertensive patients compared to non-hypertensive patients prior to the occurrence of structural retinal vascular remodeling. The correlation between RVR and WLR indicates that RVR is a reliable, non-invasive and early-sensitive marker of vascular remodeling in early hypertension.
Introduction
Vascular and endothelial function of the vasculature structure is impaired in patients with primary hypertension at very early stages [Citation1–3]. Scanning laser Doppler flowmetry (SLDF) offers the opportunity to analyze retinal arteriolar structural parameters (vessel and lumen diameters, wall thickness, wall to lumen ratio, wall cross section area) and the retinal perfusion (retinal capillary flow) non-invasively, in vivo in humans, and with a good reproducibility [Citation1,Citation4–6]. In accordance with changes in the systemic circulation several studies revealed that increased wall-to-lumen-ratio (WLR) in retinal arterioles reflects subclinical organ damage and serves as a predictor for impaired cardiovascular outcome [Citation7–9].
Increased peripheral resistance (TPR) is the hemodynamic hallmark of arterial hypertension as already described by Folkow in 1971 [Citation10]. TPR is determined predominantly (i.e. approximately 90% of its variance) by structural and functional components of small resistance vessels in the peripheral circulation. Similar to the changes of resistance vessels in the systemic circulation, vascular remodeling in the retinal circulation could lead to elevated retinal vascular resistance (RVR). Thereof the question arose whether at an early stage of hypertension changes can be measured in the smallest resistance vessels to detect early changes of the vascular structure in the retinal circulation [Citation11].
The aim of the current study was to investigate early hemodynamic markers of arterial hypertension in the retinal circulation and compare them to early arteriolar structural parameters that indicate vascular remodeling, nowadays also measurable non-invasively and with high quality. Retinal vascular resistance (RVR) is derived from hemodynamic parameters as mean arterial pressure (MAP) divided by retinal capillary flow (RCF) [RVR = MAP/RCF].
The opportunity to measure non-invasively retinal perfusion and to determine vascular resistance of retinal small vessels may also serve as an useful model to obtain insight into vascular function of cerebral circulation, because the retinal vasculature is morphologically and functionally related to cerebral circulation due to the common origin of the retinal and cerebral arterial tree from the internal carotid artery. Clinical data support further that structural changes of small arteries in the retinal circulation mirror those from the cerebral circulation [Citation12,Citation13].
Material and methods
Study design and study population
In this analysis of functional and structural parameters of retinal arterioles hypertensive patients and normotensive subjects were included in various randomized controlled trials. All subjects had the same study protocol at baseline, all measurements took place at the Clinical Research Center of the Department of Nephrology and Hypertension, University of Erlangen-Nuremberg, Germany (www.crc-erlangen.de). Participants were recruited by advertising in local newspapers in the area of Erlangen-Nürnberg, Germany, and eligible subjects were enrolled consecutively. Written informed consent was obtained before study inclusion. The study protocol of each trial was approved by the Local Ethics Committee (University of Erlangen-Nürnberg), and the studies were conducted in accordance with the Declaration of Helsinki and the principles of good clinical practice guidelines. The financial sponsor did not contribute to data collection, interpretation of the data, or the decision to approve and submit the manuscript.
Patients with arterial hypertension (defined as stage 1 [Citation14] after appropriate wash-out of antihypertensive therapy) and normotensive individuals (defined by systolic blood pressure <140 mmHg and diastolic blood pressure <90 mmHg), of male gender, age between 19 and 74 years, and non-smokers without any cardiovascular disease were included [Citation15,Citation16] (www.clinicaltrials.gov: NCT00627952 and NCT01318395). In addition to study specific exclusion criteria, common key exclusion criteria were the presence of glaucoma, optic nerve atrophy and diabetes mellitus.
No dilatation of the pupil is required to perform the retinal measurements[Citation5,Citation17], as this could distort the measured values of RCF [Citation18]. All examinations of the retinal vasculature were performed in routine conditions: sitting position after 15 min of rest, in a darkened room, between 8 am and 2 pm after a light breakfast (before lunch) [Citation5].
Immediately before the SLDF measurement, mean arterial pressure (MAP) was measured three times in each subject on the arm with higher blood pressure in a sitting position with an oscillometric device (DINAMAP Pro 100 V2; GE Medical Systems/USA). The last of three measurements after 10 minutes of rest was selected for the calculation of RVR.
Assessment of retinal capillary flow (RCF)
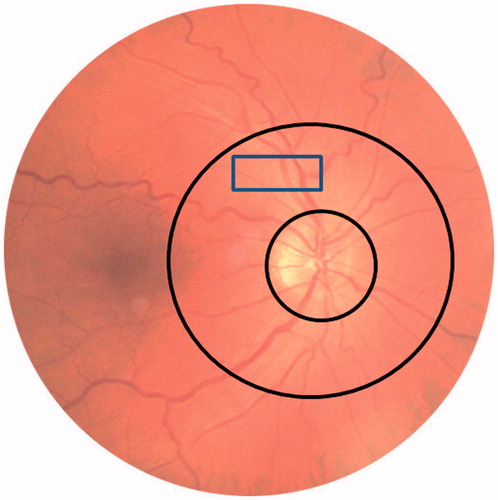
Using SLDF by Heidelberg Retina Flowmetry at 670 nm (Heidelberg Engineering GmbH/Germany) a retinal sample of length 2.56 mm (256 points) x height 0.64 mm (64 lines) x depth 0.30 mm (128 lines – the values were averaged for each line and point automatically from 3 dimensional scanning into 2 dimensional image) was scanned within 2 seconds at a pixel resolution of 10 × 10 µm [Citation17]. Measurements were performed in the juxtapapillary area of the right eye, 2 to 3 mm temporally superior to the optic nerve (). Analysis of perfusion images was performed offline with automatic full-field perfusion imaging analysis (AFFPIA) [Citation5,Citation19]. This led to a perfusion map excluding vessels with a diameter of >20 µm, without saccades, and without pixels with inadequate reflectivity. The mean retinal capillary flow was calculated in the scanned area along the retinal arteriole automatically and expressed in arbitrary units. The average from 3 such measurements was taken.
By capturing the mean arterial pressure immediately before the SLDF measurement and the calculation of the mean retinal capillary flow by using AFFPIA, the vascular resistance of the retinal arterioles was calculated by the formula: RVR = MAP/RCF as a hemodynamic parameter of vascular changes in the retinal circulation. Of note, not the total retinal flow was assessed but the flow in a well-defined scanned area of the same size at the same anatomical site for all participating subjects. Due to this approach RCF is given in arbitrary units (AU) and not in ml/min.
Assessment of parameters of retinal arterioles
All parameters of the retinal arterioles were assessed using SLDF and analyzed by AFFPIA program Version 4.10. . An arteriole with a size between 80 µm and 140 µm was scanned on the same image as used for RCF measurement and used for analysis [Citation5]. During one heart beat (one systole and one diastole visualized and selected from the flow-image). The outer vessel diameter (OD) was measured in reflection images and the inner diameter (ID) in flow images. These assessments allow the calculation of the structural parameters of retinal arterioles:
Wall-to-Lumen Ratio WLR = (OD-ID)/ID
Wall Thickness WT = (OD-ID)/2
Wall Cross-Section Area WCSA = (π/4)×(OD2-ID2)
The reliability of the measurements was described before, overall variation of coefficient were less than 10% with the exception of the wall cross-sectional area (12.5%) [Citation5].
Statistical analysis and prestudy
All statistical analyses were performed using IBM® SPSS® Statistics Version 22 (IBM Corporation Armonk, New York/USA). Significant deviations from normal distribution were excluded by the Kolmogorov-Smirnov test. Comparisons of parameters with normal distribution were performed by using student t-tests, and with not-normally distributed parameters by using Mann-Whitney-U-Test, respectively. Correlation analyses were performed calculating Pearson´s correlation-coefficient. Multiple regression analysis and analysis of covariance, respectively, were used to take confounding variables into account. A two-tailed p < .05 was considered to be significant.
Adjustment for potential confounders
To corroborate our findings with respect to RVR, we adjusted for potential confounders in two different models. We first adjusted for all variables for which a significant difference between the two groups existed (i.e. age and BMI) and then we fully adjusted for all major cardiovascular risk factors including age, BMI, HDL- and LDL-cholesterol. All subjects were male, were non-smokers and had no diabetes or cardiovascular disease.
Test-retest reliability of RVR was assessed in six volunteers from clinical staff by using SLDF three times within six weeks. The method of measuring retinal perfusion and structural parameters and the calculation of structural parameters of the retinal arterioles were described above [Citation5,Citation17,Citation19] The analysis of the RVR measurement reliability revealed a coefficient of variation of 7.75 ± 2.11% and Cronbach´s alpha was 0.90, thereby demonstrating excellent test-retest reliability.
Results
Clinical characteristics
Clinical characteristic of 45 normotensive (NT) and 123 hypertensive (HT) patients is comprised and compared in . All were males, non-smokers and had no cardiovascular disease or diabetes mellitus. There was no significant difference in lipid levels between NT and HT patients (triglycerides, total cholesterin, HDL-cholesterin and LDL-cholesterin). There was also no significant difference in renal function or albuminuria between NT and HT subjects. Only the clinical parameters age and BMI showed a significant difference between the two groups. Of note, we did not find a correlation between age and RVR in the whole study population as well as in NT and HT subjects.
Table 1. Clinical parameters.
Retinal parameters
There was a significant difference in the RCF between HT and NT patients (p = .023). Regarding the structural parameters, the HT group did not show vascular remodeling of retinal arterioles compared to the NT group, since WLR and WCSA were similar in HT and NT subjects. Lumen diameter was lower in the HT group (p = .001), corresponding to the slightly lower value for RCF in HT patients ().
Table 2. Retinal parameters.
Vascular resistance of retinal vessels (RVR)
The parameter RVR was significantly (p < .001) () higher in the HT group than in the NT group (). As age and BMI have been found to be different between the groups, adjustment has been made for the two variables. After adjustment for the 2 clinical parameters, RVR was significantly different between the two groups (p < .001) and after additional adjustment of HDL- and LDL-cholesterol RVR was still significantly higher in the hypertensive group (p < .001).
Correlation of RVR with WLR
There was a significant correlation of RVR with WLR in the total study population (r = 0.271, p < .001) (), in the subgroup of HT subjects (r = 0.26, p = .004), and a trend was observed in NT subjects (r = 0.25, p = .09) thereby indicating that the RVR is associated with eutrophic vascular remodeling in the retinal arterioles. Similarly, we observed a significant correlation of WLR with age in the total study population (r = 0.217, p = .005) and in HT subjects (r = 0.193, p = .034).
After adjusting for BMI and age, the partial correlation of RVR and WLR was still significant (r = 0.287, p < .001). Even after adjusting for other cardiovascular risk factors (age, BMI, HDL- and LDL-cholesterol) the correlation of RVR and WLR was significantly higher (r = 0.272, p = .001).
Discussion
Change in total peripheral resistance is long known as hemodynamic hallmark of hypertensive disease, mainly referred to changes in resistance vessels (arterioles) in the periphery, whose diameter is between 100-300 µm [Citation20,Citation21]. We applied this concept to retinal circulation and found that retinal vascular resistance (RVR) derived from hemodynamic parameters detected changes in the vascular resistance of the microvascular bed due to arterial hypertension prior to the detection of changes in vascular remodeling (e.g. WLR) [Citation1].
We therefore suggest RVR (retinal vascular resistance) as new, non-invasive marker of very early functional vascular changes in hypertension that deserves further rigorous examinations [Citation5,Citation17,Citation19]. Until now, early structural changes of arteries and arterioles were detected by measuring increased media-to-lumen-ratio, media thickness and media cross-sectional area in either isolated subcutaneous resistance vessels in patients with arterial hypertension compared to non-hypertensive patients [Citation21], or non-invasively in retinal arterioles by using scanning laser doppler flowmetry or adaptive optics [Citation1,Citation7,Citation9,Citation22,Citation23]. In particular increased wall-to-lumen ratio (WLR) is one of the most reliable parameter that early indicate changes in arterioles due to hypertension [Citation9,Citation23] independently of age that per se influences WLR as well [Citation24,Citation25]. The results of our study did not reveal any significant vascular remodeling (no difference in WLR or WCSA) between hypertensive and normotensive individuals, but of note our patients had hypertension stage 1 with antihypertensive therapy and not advanced hypertensive disease. In contrast, both components of the parameter RVR were significantly different between the two groups. Interestingly, we observed a significant correlation of RVR with WLR in our study population supporting our concept that RVR is a valid marker of microvascular remodeling in the retinal circulation [Citation22,Citation26].
Which pathogenic mechanism is causing this increase of RVR in the very early stage of hypertensive disease? We observed the difference in the retinal capillary blood flow (RCF) between HT and NT patients without any difference in vascular remodeling (e.g. WLR or WCSA). In accordance, hypertensive patients revealed a decreased lumen diameter compared to the normotensive subjects. It is known that subjects in the early stage of arterial hypertension display a smaller inner diameter of subcutaneous arterioles caused by rearrangement of smooth muscle cells, without any marked growth response, that in turn may increase vascular resistance [Citation27,Citation28]. In addition we suggest that rarefaction of arterioles represents another reason for the increase of RVR and the decrease of RCF. It has previously been shown that vascular resistance and perfusion is affected by changes of capillary density [Citation29] and we most recently observed greater capillary rarefaction in hypertensive patients compared to normotensive individuals [Citation30]. The pathophysiological reason of retinal capillary rarefaction is a loss of pericytes, that leads to vulnerable capillaries, and exaggerated capillary contraction with the study consequence of reduced perfusion [Citation31,Citation32]. In the current study we observed a reduced retinal capillary flow in the hypertensive group.
Since cerebral and retinal arterioles disclose a similar pattern of vascular remodeling in hypertensive patients and stem both from the A. carotis interna with similar histological profile, the examination of retinal arterioles with SLDF might be a scientific tool to assess early vascular changes in the cerebral circulation and useful to stratify risk groups for incident cerebrovascular events. The concept that retinal circulation mirrors needs to be further consolidated [Citation12,Citation33] but the advantage in that the retinal vascular bed can be directly observed non-invasively and in vivo in humans.
Thus, our data suggest a new tool to assess vascular changes in the retinal (and potentially cerebrovascular) bed in hypertension that deserves further examinations.
Disclosure statement
The authors report no conflicts of interest.
References
- Lehmann MV, Schmieder RE. Remodeling of retinal small arteries in hypertension. Am J Hypertens. 2011;24:1267–1273.
- Park JB, Schiffrin EL. Small artery remodeling is the most prevalent (earliest?) form of target organ damage in mild essential hypertension. J Hypertens. 2001;19:921–930.
- Schiffrin EL, Deng LY. Relationship between small-artery structure and systolic, diastolic and pulse pressure in essential hypertension. J Hypertens. 1999;17:381–387.
- Michelson G, Welzenbach J, Pal I, et al. Functional imaging of the retinal microvasculature by scanning laser Doppler flowmetry. Int Ophthalmol. 2001;23:327–335.
- Harazny JM, Raff U, Welzenbach J, et al. New software analyses increase the reliability of measurements of retinal arterioles morphology by scanning laser Doppler flowmetry in humans. J Hypertens. 2011;29:777–782.
- Kreis AJ, Nguyen T, Rogers S, et al. Reliability of different image analysis methods for scanning laser Doppler flowmetry. Curr Eye Res. 2008;33:493–499.
- Harazny JM, Ritt M, Baleanu D, et al. Increased wall:lumen ratio of retinal arterioles in male patients with a history of a cerebrovascular event. Hypertension. 2007;50:623–629.
- Izzard AS, Rizzoni D, Agabiti-Rosei E, et al. Small artery structure and hypertension: adaptive changes and target organ damage. J Hypertens. 2005;23:247–250.
- Rizzoni D, Agabiti-Rosei E. Structural abnormalities of small resistance arteries in essential hypertension. Intern Emerg Med. 2012;7:205–212.
- Folkow B. Regulation of the peripheral circulation. Br Heart J. 1971;33(Suppl):27–31.
- Agabiti-Rosei E. Structural and functional changes of the microcirculation in hypertension: influence of pharmacological therapy. Drugs. 2003;63:19–29.
- Wong TY, Klein R, Nieto FJ, et al. Retinal microvascular abnormalities and 10-year cardiovascular mortality: a population-based case-control study. Ophthalmology. 2003;110:933–940.
- Tso MO, Jampol LM. Pathophysiology of hypertensive retinopathy. Ophthalmology. 1982;89:1132–1145.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC). J Hypertens. 2013;31:1925–1938.
- Ott C, Schneider MP, Raff U, et al. Effects of manidipine vs. amlodipine on intrarenal haemodynamics in patients with arterial hypertension. Br J Clin Pharmacol. 2013;75:129–135.
- Jumar A, Ott C, Kistner I, et al. Effect of aliskiren on vascular remodelling in small retinal circulation. J Hypertens. 2015;33:2491–2499.
- Michelson G, Schmauss B, Langhans MJ, et al. Principle, validity, and reliability of scanning laser Doppler flowmetry. J Glaucoma. 1996;5:99–105.
- Harazny JM, Schmieder RE, Welzenbach J, et al. Local application of tropicamide 0.5% reduces retinal capillary blood flow. Blood Press. 2013;22:371–376.
- Michelson G, Welzenbach J, Pal I, et al. Automatic full field analysis of perfusion images gained by scanning laser Doppler flowmetry. Br J Ophthalmol. 1998;82:1294–1300.
- Folkow B. Physiological aspects of primary hypertension. Physiol Rev. 1982;62:347–504.
- Aalkjaer C, Eiskjaer H, Mulvany MJ, et al. Abnormal structure and function of isolated subcutaneous resistance vessels from essential hypertensive patients despite antihypertensive treatment. J Hypertens. 1989;7:305–310.
- Ritt M, Schmieder RE. Wall-to-lumen ratio of retinal arterioles as a tool to assess vascular changes. Hypertension. 2009;54:384–387.
- Rosenbaum D, Mattina A, Koch E, et al. Effects of age, blood pressure and antihypertensive treatments on retinal arterioles remodeling assessed by adaptive optics. J Hypertens. 2016;34:1115–1122.
- Meixner E, Michelson G. Measurement of retinal wall-to-lumen ratio by adaptive optics retinal camera: a clinical research. Graefes Arch Clin Exp Ophthalmol. 2015;253:1985–1995.
- Bruno RM, Duranti E, Ippolito C, et al. Different impact of essential hypertension on structural and functional age-related vascular changes. Hypertension. 2017;69:71–78.
- Heagerty AM, Aalkjaer C, Bund SJ, et al. Small artery structure in hypertension. Dual processes of remodeling and growth. Hypertension. 1993;21:391–397.
- Schiffrin EL. Remodeling of resistance arteries in essential hypertension and effects of antihypertensive treatment. Am J Hypertens. 2004;17:1192–1200.
- Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38:581–587.
- Antonios TFT, Singer DRJ, Markandu ND, et al. Rarefaction of skin capillaries in borderline essential hypertension suggests an early structural abnormality. Hypertension. 1999;34:655–658.
- Jumar A, Harazny JM, Ott C, et al. Improvement in Retinal Capillary Rarefaction After Valsartan Treatment in Hypertensive Patients. J Clin Hypertens. 2016;18:1112–1118.
- Schrimpf C, Teebken OE, Wilhelmi M, et al. The role of pericyte detachment in vascular rarefaction. J Vasc Res. 2014;51:247–258.
- Goligorsky MS. Microvascular rarefaction: the decline and fall of blood vessels. Organogenesis. 2010;6:1–10.
- Witt N, Wong TY, Hughes AD, et al. Abnormalities of retinal microvascular structure and risk of mortality from ischemic heart disease and stroke. Hypertension. 2006;47:975–981.