Abstract
Whilst much uncertainty exists as to the efficacy of renal denervation (RDN), the positive results of the DENERHTN study in France confirmed the interest of an economic evaluation in order to assess efficiency of RDN and inform local decision makers about the costs and benefits of this intervention. The uncertainty surrounding both the outcomes and the costs can be described using health economic methods such as the non-parametric bootstrap. Internationally, numerous health economic studies using a cost-effectiveness model to assess the impact of RDN in terms of cost and effectiveness compared to antihypertensive medical treatment have been conducted. The DENERHTN cost-effectiveness study was the first health economic evaluation specifically designed to assess the cost-effectiveness of RDN using individual data. Using the DENERHTN results as an example, we provide here a summary of the principle methods used to perform a cost-effectiveness analysis.
Background
Catheter-based denervation of the renal arteries has emerged as a potential treatment for resistant hypertension [Citation1–3]. The initial observational studies and open-label randomized controlled trial [Citation4,Citation5], have shown large reductions in office blood pressure (BP) at 6 months after renal denervation (RDN). However, several limitations of these studies, including non-randomization, limited assessment of ambulatory BP, lack of blinding, or lack of a sham procedure as a control, make broad application of the findings unreliable. Simplicity-HTN3 study, a prospective, single-blind, randomized, sham-controlled trial, showed no significant difference in office and ambulatory systolic blood pressure (SBP) at 6 months between the RDN and the sham group; however several methodological concerns were raised, potentially contributing to this neutral result [Citation6]. The Renal Denervation for Hypertension (DENERHTN) trial, a French study, was carefully designed to overcome some of these methodological shortcomings and compared RDN with the radiofrequency Symplicity™ catheter and standardized stepped-care antihypertensive treatment (SSAHT) with SSAHT alone. This study reported a decrease of 15.8 mmHg in daytime ambulatory SBP in the group that underwent RDN versus a decrease of 9.9 mmHg in the group receiving SSAHT alone after 6 months follow-up (p = .033) [Citation7]. Between 2014 and 2017, eight other international trials on RDN were published [Citation8–15]. Among them, a 2017 multicenter, international, single-blind, randomized, sham controlled trial on RDN in 80 patients with uncontrolled mild to moderate hypertension without antihypertensive medications (SPYRAL HTN-OFF MED) showed a statistically significant between-groups difference of 5 mmHg in 24-hour SBP in favor of RDN [Citation15]. On the other hand, a recent systematic meta-analysis of 10 of these international trials showed that overall there was no significant effect on 24-hour SBP between the RDN and the control group (difference between group of −1 mmHg in favor of RDN, p = .45) but there was significant heterogeneity [Citation16]. Only three studies in this meta-analysis had significant results between the two groups: the French DENERHTN trial [Citation7], with a difference of 5.9 mmHg in 24-hour SBP in favor of RDN and a Norwegian study [Citation9] and a Spanish study [Citation13] with respectively a difference of 11 mmHg and 17.9 mmHg in 24-hour SBP in favor of drug treatment.
Internationally, numerous health economic studies using a cost-effectiveness model to assess the impact of RDN in terms of cost and effectiveness compared to antihypertensive medical treatment have been conducted [Citation17–22]. In all of these studies the effectiveness was considered to be the gain in quality of life years (QALY) and they are presented in the Online Supplement.
The DENERHTN study included an economic evaluation carried out alongside the trial. All economic data were prospectively collected in parallel with the clinical data. Whilst much uncertainty exists as to the efficacy of RDN, the positive results of the DENERHTN study in France and the availability of the DENERHTN individual data confirmed the interest of an economic evaluation to evaluate the joint distribution of costs and benefits and thus addressing issues of efficiency, that is, whether or not the intervention could be a good use of health-care resources.
Economic evaluation challenges
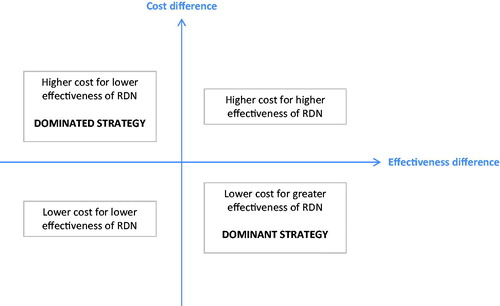
The final outcome of the RDN economic studies was expressed in an incremental cost-effectiveness ratio (ICER) which is a composite of two variables, cost and outcome. This ratio represents the cost for one additional unit of effectiveness, which is the clinical trial result, i.e. the BP lowering efficacy, of the new strategy (RDN + SSAHT) compared with the reference (SSAHT). The conclusions of the trial and the economic study complement one another since the efficacy measure used in the economic analysis may not always be the primary outcome measure used in the trial. In addition, the statistical methods may differ, with clinical trials generally being assessed on the basis of statistical significance for superiority or non-inferiority for the primary outcome and economic evaluations being assessed on the probability that an intervention is cost-effective [Citation23]. The probability that the intervention is cost-effective is often assessed by the non-parametric bootstrap method which makes multiple estimates of the ICER by randomly re-sampling the patient population to create sub-samples. The set of estimated ICERs are presented as a cloud of points on a cost-effectiveness plane which is used to visually represent the differences in costs and health outcomes between treatment alternatives in two dimensions, by plotting the costs against effects on a graph (). The percentage of ICERs in each part of the cost-effectiveness plane is then presented in order to know where the RDN strategy is mostly located. If a new strategy is dominated (in the top left-hand quadrant) it will be rejected and on the contrary, if it is dominant (in the bottom right-hand quadrant) it will be accepted by the public decision-maker. If the new strategy is located in the top right-hand quadrant or in the bottom left-hand quadrant, indicating respectively a higher cost for greater effectiveness or a lower cost for lower effectiveness, a threshold will have to be set by the public decision-maker [Citation24]. This threshold corresponds to a maximum monetary value that a decision-maker might be willing to pay for a unit change in outcome and can be visualized on a cost-effectiveness acceptability curve that is derived from the re-sampling. This shows the probability that the treatment is cost-effective compared with the alternative according to the value of the threshold [Citation25].
In hypertension studies the clinical effectiveness has usually been assessed by the change in SBP in the short-term, which is a validated surrogate outcome. For the purpose of hypertension economic evaluations, the SBP was extrapolated beyond the trial to final endpoints such as avoided events (cardiovascular diseases, renal insufficiency etc.) or quality-adjusted life-year (QALY) with the assumption that the decrease in SBP would be maintained in the long-term and that SBP reduction with RDN was associated with the same decrease in events as reduction induced by drug treatment in the course of randomized trials [Citation26]. Both assumptions were disputed by the fact that effectiveness of a drug investigated in a trial tends to be higher than in real life [Citation27,Citation28]. Numerous cardiovascular (CV) risk prediction formulas have been developed to quantify the 10-year risk of CV death or events, such as the SCORE risk function [Citation29], the Framingham risk function [Citation30] or the JBS3 CV risk assessment model [Citation31]. The model to be used should be chosen according to the study and the study population.
Example of the DENERHTN cost-effectiveness study
To inform healthcare payers in France, we performed a health economic analysis on the DENERHTN study clinical results.
Data for the DENERHTN cost-effectiveness study were prospectively and concurrently collected during the trial, in accordance with the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement [Citation32]. The cost-effectiveness of RDN with SSAHT compared to SSAHT alone, defined as the additional cost per mmHg reduction in daytime ambulatory SBP, was determined. Only direct costs were taken into account in this economic evaluation as recommended by the French National Authority for Health (HAS) [Citation33]. Both hospital and non-hospital resources were considered (). The analysis was conducted from the French healthcare perspective (compulsory health insurance, complementary health insurance and patient co-payments) using tariffs with the exception of RDN for which hospital production costs were used as a proxy for a tariff. The time horizon was 6 months.
Table 1. Costs collected for the DENERHTN economic study.
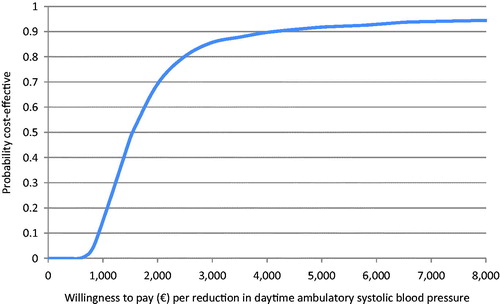
The average cost of RDN initial hospitalization was estimated at €8 492. The average total cost at 6 months was €8 528 higher in the RDN group (p < .0001) and the difference between the two groups in average reduction in daytime ambulatory SBP was 5.9 mmHg (p = .033). The ICER was €1 450/mmHg reduction in BP. The scatter plot of the probabilistic bootstrapping had 94% of the replications in the top right-hand quadrant, indicating a higher cost for greater effectiveness of RDN (). In addition, the acceptability curve showed that at a threshold of €1 540/mmHg reduction in daytime ambulatory SBP between the baseline and 6 months there was 50% chance that the RDN treatment was cost-effective ().
Figure 2. Incremental cost and effectiveness of RDN added to a SSAHT compared to SSAHT alone: cost effectiveness plane.
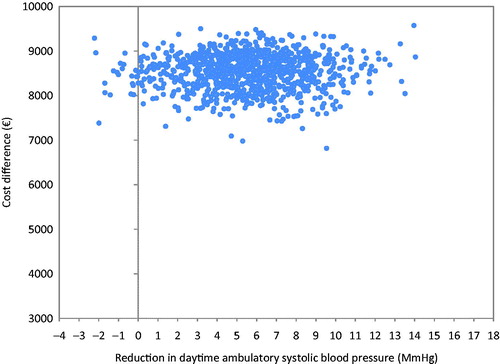
Figure 3. Cost-effectiveness acceptability curve showing the probability that RDN added to a SSAHT is cost-effective compared to SSAHT alone.
The ICER of €1 450/mmHg reduction in BP was robust since it was based on data collected prospectively, yet interpretation of this result in terms of real world cost and benefit may not be intuitive. It is generally agreed that lowering BP in hypertensive patients reduces CV morbidity and mortality [Citation34]. In order to project the expected reduction of CV morbidity and mortality in the long term, it is necessary to carry out efficiency analyses beyond the usual short-term follow-up period of a trial. We carried out two efficiency analyses in the DENERHTN trial to assess the cost-effectiveness of RDN with SSAHT compared to SSAHT alone. The first one used the SCORE risk function from the European cardiovascular disease risk assessment model [Citation29]. The average risk of cardiovascular death at 10 years was 3.0% (95% CI 2.0 to 4.0) for a 6-months cost of €9 253 in the RDN group and 3.6% (95% CI 2.6 to 4.5) for a 6-months cost of €725 in the control group (p = .375). The ICER was estimated at €1 375 304/cardiovascular death avoided at 10 years. Which means that to save one life in 10 years’ time, 167 people would have to be treated with RDN at a 6-months cost of €1.4 million. This cost can be offset by the reduced cost of hospitalizations for severe CV events impacting the healthcare system. However, the SCORE risk function estimates the risk of CV death at a given time without taking into account the evolution of BP between inclusion and 6 months follow-up. Thus further analysis was carried out based on the relative risk (RR) of CV events using the results of a meta-regression analysis showing the RR reductions proportional to the magnitude of the BP reductions achieved in 123 studies with 613,815 participants [Citation35]. We estimated the baseline risk of CV events at 10 years with the Framingham risk function from the American cardiovascular disease risk assessment model for our study population [Citation30]. Using the RR reduction in major CV events regressed against the difference in achieved SBP from the meta-analysis we calculated the RR of CV events at 10 years for each arm. The average RR of CV events at 10 was 15.5% (95% CI 12.1 to 18.8) for a 6-months cost of €9 253 in the RDN group and 17.6% (95% CI 14.4 to 20.7) for a 6-months cost of €725 in the control group (p = .367). The cost-effectiveness of RDN with SSAHT compared to SSAHT alone was finally estimated at €408 021/patient avoiding CV event(s) at 10 years.
The DENERHTN cost-effectiveness study was the first health economic evaluation using clinical data prospectively collected from an open-label, randomized controlled trial with blind endpoint evaluation. Moreover, the international health economic studies [Citation17–22] have estimated that RDN is cost-effective using the much larger office BP results reported in an early randomized controlled trial or observational studies and none of these studies were specifically designed to assess cost-effectiveness, whereas DENERHTN was specifically designed to assess the cost-effectiveness of RDN using individual data and thus should have more robust economic results. The international modeling study results are not directly comparable to our study because the effectiveness criterion was the QALY, costs were calculated on the entire life and depend on the country setting. In the case of DENERHTN, the positive clinical efficacy results were shown to potentially translate into long term health gains.
Conclusion
Whilst much uncertainty exists as to the efficacy of RDN, the positive results of the DENERHTN study confirmed the interest of an economic evaluation to inform healthcare payers in France. Indeed, the uncertainty surrounding both the outcomes and the costs can be described using health economic methods such as the non-parametric bootstrap. Furthermore, in hypertension studies the clinical effectiveness has usually been assessed by the change in SBP on the short-term, which is a validated surrogate outcome. In economic evaluations, the SBP can be extrapolated beyond the trial to final endpoints such as avoided events (cardiovascular diseases, renal insufficiency etc.) or quality-adjusted life year (QALY) which will facilitate public decision-making.
The DENERHTN economic study showed that RDN with SSAHT is more expensive but also more effective than SSAHT alone at 6 months and the scatter plots of the probabilistic bootstrapping confirm this conclusion with the majority of the replications in the upper right quadrant whatever the effectiveness criterion (BP reduction, risk of CV events or death). However, we do fully acknowledge that the efficacy of DENERHTN is not reflected in other clinical studies and that it would be misleading to conclude that renal denervation is cost effective from only one study given the multiple uncertainties. Whilst our results are not the definitive answer to the RDN cost-effectiveness question, it serves as an example and explanation of how a prospective economic analysis can be conducted with more precision than those based on modeling alone. However, the positive results of the proof-of-concept SPYRAL HTN-OFF MED trial may lead to further research on the interest of RDN in uncontrolled hypertension [Citation15].
Collaborators/investigators
Collaborators: H PEREIRA, I HAMDIDOUCHE
Investigators (with the number of patients enrolled and randomized at each center given in parentheses) and committees participated in the DENERHTN trial: Hôpital Européen Georges Pompidou, Paris (31/28) – L Amar, G Bobrie, A Lorthioir, M Monge, J-Y Pagny, PF Plouin, M Sapoval; Hôpital Cardiologique, Lille (20/15) – G Claisse, P Delsart, M Midulla, C Mounier-Vehier; Hôpital de la Croix Rousse and Hôpital Edouard Herriot, Lyon (14/13) – PY Courand, R Dauphin, JP Fauvel, P Lantelme, O Rouvière; Hôpital Saint André and Hôpital Pellegrin, Bordeaux (14/13) – A Cremer, P Gosse, N Grenier, Y Lebras, H Trillaud; Hôpital Arthur Gardiner, Dinard and CHU Rennes (12/12) – T Denolle, C Dourmap-Collas, JF Heautot, A Larralde, F Paillard; Hôpital de la Pitié Salpétrière, Paris (6/5) – P Cluzel, X Girerd, D Rosenbaum; Hôpital Bretonneau, Tours (5/4) – D Alison, JM Halimi; CHU Nancy-Brabois, Nancy (4/1) – M Claudon, B Popovic, P Rossignol, F Zannad; CHU de Grenoble, Grenoble (3/3) – JP Baguet, O Ormezzano, F Thony; CHU de la Timone, Marseille (3/3) – JM Bartoli, B Vaïsse; Hôpital la Milétrie, Poitiers (3/3) – J Drouineau, D Herpin, P Sosner, JP Tasu, S Velasco; Hôpital Lapeyronie and Hôpital Arnaud de Villeneuve, Montpellier (2/2) – J Ribstein, H Vernhet-Kovacsik; CHU Rangueil, Toulouse (2/2) – B Bouhanick, B Chamontin, H Rousseau; Hôpital Avicenne, Bobigny (1/1) – S Le Jeune, M Lopez-Sublet, JJ Mourad; Hôpital Pasteur, Nice (1/1) – L Bellmann, V Esnault, E Ferrari, Scientific Committee – M Azizi (chair), M Sapoval (cochair), G Bobrie, G Chatellier, PF Plouin, F Zannad, JM Halimi, JP Baguet, H Vernhet-Kovacsik, I Durand-Zaleski Data Safety Committee – JP Beregi (chair), M Lièvre, A Persu.
Online_supplement.docx
Download MS Word (22.5 KB)Disclosure statement
J Bulsei, M Darlington and I Durand-Zaleski have no conflict of interest. M Azizi has received honoraria for advisory board meetings from Vessix, Boston Scientific Corporation, Cordis, Actelion, has received speakers’ honoraria from Cordis, CVRx, Servier; was involved as investigator in Symplicity HTN-2 (Ardian/Medtronic) and Reduce-HTN (Vessix/Boston Scientific Corporation) trials; has received a research grant from Servier.
Additional information
Funding
References
- Mahfoud F, Lüscher TF, Andersson B, et al. Expert consensus document from the European Society of Cardiology on catheter-based renal denervation. Eur Heart J. 2013;34:2149–2157.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–1357.
- Schmieder RE, Redon J, Grassi G, et al. ESH position paper: renal denervation: an interventional therapy of resistant hypertension. J Hypertens. 2012;30:837–841.
- Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281.
- Symplicity HTN-2 Investigators, Esler MD, Krum H, et al. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903–1909.
- Bhatt DL, Kandzari DE, O’Neill WW, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–1401.
- Azizi M, Sapoval M, Gosse P, et al. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet Lond Engl. 2015;385:1957–1965.
- Rosa J, Widimský P, Toušek P, et al. Randomized comparison of renal denervation versus intensified pharmacotherapy including spironolactone in true-resistant hypertension: six-month results from the Prague-15 study. Hypertens (Dallas Tex 1979). 2015;65:407–413.
- Fadl Elmula FEM, Hoffmann P, Larstorp AC, et al. Adjusted drug treatment is superior to renal sympathetic denervation in patients with true treatment-resistant hypertension. Hypertens (1979). 2014;63:991–999.
- Desch S, Okon T, Heinemann D, et al. Randomized sham-controlled trial of renal sympathetic denervation in mild resistant hypertension. Hypertens (1979). 2015;65:1202–1208.
- Kario K, Ogawa H, Okumura K, et al. SYMPLICITY HTN-Japan: first randomized controlled trial of catheter-based renal denervation in asian patients. Circ J. 2015;79:1222–1229.
- Mathiassen ON, Vase H, Bech JN, et al. Renal denervation in treatment-resistant essential hypertension. A randomized, SHAM-controlled, double-blinded 24-h blood pressure-based trial. J Hypertens. 2016;34:1639–1647.
- Oliveras A, Armario P, Clarà A, et al. Spironolactone versus sympathetic renal denervation to treat true resistant hypertension: results from the DENERVHTA study: a randomized controlled trial. J Hypertens. 2016;34:1863–1871.
- de Jager RL, de Beus E, Beeftink MMA, et al. Impact of medication adherence on the effect of renal denervation: the SYMPATHY trial. Hypertens (1979). 2017;69:678–684.
- Townsend RR, Mahfoud F, Kandzari DE, etet al. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet Lond Engl. 2017;Aug 25. [Epub ahead of print].
- Fadl Elmula FEM, Feng Y-M, Jacobs L, et al. Sham or no sham control: that is the question in trials of renal denervation for resistant hypertension. A systematic meta-analysis. Blood Press. 2017;26:195–203.
- Henry TL, De Brouwer BFE, Van Keep MML, et al. Cost-effectiveness of renal denervation therapy for the treatment of resistant hypertension in The Netherlands. J Med Econ. 2015;18:76–87.
- Tilden D, McBride M, Whitbourn R, et al. Cost effectiveness of catheter-based renal denervation for treatment resistant hypertension: an Australian payer perspective. Value Health. 2014;17:A762.
- Gladwell D, Henry T, Cook M, et al. Cost effectiveness of renal denervation therapy for the treatment of resistant hypertension in the UK. Appl Health Econ Health Policy. 2014;12:611–622.
- Kontsevaia AV, Suvorova EI, Khudiakov MB. [Economic efficiency of renal denervation in patients with resistant hypertension: results of Markov modeling]. Kardiologiia. 2014;54:41–47.
- Dorenkamp M, Bonaventura K, Leber AW, et al. Potential lifetime cost-effectiveness of catheter-based renal sympathetic denervation in patients with resistant hypertension. Eur Heart J. 2013;34:451–461.
- Geisler BP, Egan BM, Cohen JT, et al. Cost-effectiveness and clinical effectiveness of catheter-based renal denervation for resistant hypertension. J Am Coll Cardiol. 2012;60:1271–1277.
- Raftery J, Young A, Stanton L, et al. Theme 5: economic analysis alongside clinical trials [Internet]. NIHR Journals Library; 2015 [cited 2017 May 23]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK274338/
- Guide méthodologique pour l’évaluation économique des stratégies de santé [Internet]. Collège Économistes Santé. 2013 [cited 2017 May 19]. Available from: http://www.ces-asso.org/sites/default/files/Guide_Methodologique_CES_2003.pdf
- Fenwick E, Byford S. A guide to cost-effectiveness acceptability curves. Br J Psychiatry. 2005;187:106–108.
- Buxton MJ, Drummond MF, Van Hout BA, et al. Modelling in economic evaluation: an unavoidable fact of life. Health Econ. 1997;6:217–227.
- Pratley RE. The efficacy and effectiveness of drugs for diabetes: how do clinical trials and the real world compare? Diabetologia. 2014;57:1273–1275.
- Revicki DA, Frank L. Pharmacoeconomic evaluation in the real world. Effectiveness versus efficacy studies. Pharmacoeconomics. 1999;15:423–434.
- Conroy RM, Pyörälä K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003.
- D’Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753.
- JBS3 Board. Joint British Societies’ consensus recommendations for the prevention of cardiovascular disease (JBS3). Heart Br Card Soc. 2014;100(Suppl 2):ii1–ii67.
- Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)–explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16:231–250.
- Haute Autorité de Santé. Guide méthodologique: Choix méthodologiques pour l’évaluation économique à la HAS. [Internet]. 2011. Available from: https://www.has-sante.fr/portail/upload/docs/application/pdf/2011-11/guide_methodo_vf.pdf
- Grossman E. Blood Pressure: the lower, the better. Diabetes Care. 2011;34:S308–S312.
- Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957–967.