Abstract
Purpose: Although self-measurement of home blood pressure (HBP) is common in Japan and HBP telemonitoring via the Internet is possible, whether telemonitoring improves HBP control better than conventional practice remains unclear. Furthermore, hypertension care with online communication using telemonitored HBP is feasible, whereas the efficacy and safety of such telemedicine have not been established. We aim to compare traditional care, care with office visits using HBP telemonitoring, and antihypertensive telemedicine based on HBP telemonitoring.
Methods and design: In total, 444 patients with uncontrolled hypertension will be recruited and randomly assigned to three groups: (1) control: usual care with office visits and HBP self-report, (2) telemonitoring: weekly assessment of transmitted HBP by physicians and treatment adjustment upon office visits, or (3) telemedicine: online communication instead of office visits to adjust medication using telemonitored HBP. Primary outcome is the time to control of HBP, and secondary outcomes include achieved HBP levels, adherence, treatment intensity, adverse events, patient satisfaction and cost-effectiveness.
Discussion: Hypertension care with telemonitoring and telemedicine are expected to require shorter time to achieve HBP control compared to usual care. Combining HBP telemonitoring with telemedicine may lower the hurdles for starting and persisting to hypertension treatment and eventually reduce cardiovascular events.
Introduction and rationale
Hypertension affects an estimated 20 to 30% of the world’s adult population [Citation1,Citation2] and about half of the adult population in Japan [Citation3]. Despite the availability of various safe and effective pharmacologic therapies, the percentage of patients being treated and achieving adequate blood pressure control remains low [Citation3,Citation4]. Telemedicine provided by internet-based communication could lower the hurdle for starting and adhering to hypertension treatment, eventually leading to prevention of cardiovascular disease (CVD). However, the efficacy and safety of telemedicine without office visit for hypertension treatment have been evaluated in only a few studies [Citation5,Citation6].
Achieving target blood pressure levels in the treatment of hypertension requires patients’ adherence and persistence to their medications [Citation7], and self-measurement of home blood pressure (HBP) improves adherence to treatment and the control of blood pressure [Citation8,Citation9]. Adjusting antihypertensive medication based on self-measured HBP for long periods of time is feasible [Citation10], and HBP self-monitoring with self-titration of antihypertensive medication on individualized, pre-determined protocol is reported to result in better blood pressure control compared to usual care [Citation11,Citation12]. Telemonitoring of HBP coupled with remote pharmacist [Citation13–15] or physician management can be as efficacious and safe as usual clinical care [Citation6,Citation16,Citation17].
HBP telemonitoring combined with telemedicine may result in shorter time to blood pressure control because frequent interaction between patient and physician or management team is feasible. This approach might also result in better quality of life and higher satisfaction of patients by enabling better use of their time rather than spending much of it waiting at clinics or hospitals on condition that the technical difficulty and anxieties associated with telemedicine can be managed sufficiently.
Therefore, reduction of uncontrolled hypertension by Remote Monitoring and Telemedicine (REMOTE) Study was designed to test the effectiveness and safety of blood pressure telemonitoring and hypertension telemedicine in a prospective, randomized, open-label 3-ARM study of uncontrolled hypertensive patients.
Study design and setting
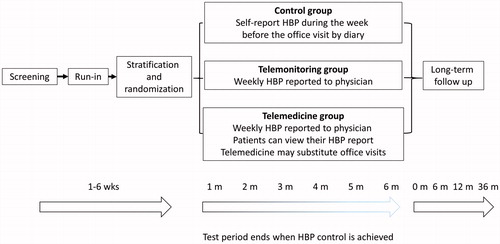
This is a multi-centered, open-label, randomized controlled trial which will address the feasibility and potential benefits of telemonitoring of HBP at centers including Women’s Medical University Hypertension Clinic and TM Clinic Nishishinjyuku. The trial consists of three groups: (1) usual care with office visits and self-report of HBP (control group), (2) office visits with weekly HBP assessment by physicians of wireless-transmitted HBP data (telemonitoring group), or (3) telemonitoring plus telemedicine with prescription by mail without office visit (telemedicine group). All patients take HBP readings using internet interfaced blood pressure monitor (HEM-7252G-HP, Omron Corporation, Kyoto), however, patients in the control and telemonitoring groups cannot review their HBP summary using the web-based automatic analysis system (MedicalLINK). The MedicalLINK system has been used successfully in previous studies [Citation18,Citation19]. Patients in the control group regularly visit physician’s office upon appointment and the physicians assess HBP by self-report in a paper diary. The study center can assess all of the HBP automatically transmitted after each measurement in all groups. In the telemonitoring group, physicians assess HBP via MedicalLINK weekly and can invite patients for an office visit if necessary. In the telemedicine group, physicians assess HBP weekly via MedicalLINK and when necessary, request patients to schedule an online interview to discuss treatment. In the telemedicine group, patients receive prescription refill or dose modification via mail.
The trial consists of 3 stages (): (i) a screening visit, at which the patient’s eligibility will be checked according to the inclusion and exclusion criteria, informed consent will be given, and the blood pressure monitoring device will be handed out; (ii) a randomized period, during which patients will be randomized in equal proportions to three groups, stratified by center, and receive antihypertensive care; (iii) follow-up period, starting at the visit when the achievement of blood pressure target is confirmed and extending up to 36 months.
Figure 1. Schematic representation of the REMOTE trial. During the run-in period, secondary hypertension is excluded and HBP level will be assessed for 7 or more days. Patients will be stratified by center during the run-in period. Intervals between office visits (and telemedicine appointments) are decided between the practitioner and the patient. The maximum duration of the treatment period is 6 months.
On 29 Aug 2017, the Ethics Committee of the Tokyo Women’s Medical University approved the protocol, which was then registered to UMIN Clinical Trials Registry on 31 Aug 2017 as UMIN000028937. The trial will start with a pilot phase at one center (TM Clinic Nishishinjuku). The pilot phase aims to assess feasibility in terms of recruitment rate as a proportion of screened patients, to test the procedures as described in the protocol, and to evaluate time-to-control of blood pressure in 15 randomized patients, of whom 5 will be treated with telemedicine, 5 will have HBP telemonitored, and 5 will be assigned to conventional treatment. The expectation is that the REMOTE protocol as piloted will roll over in the full trial without major changes.
Blood pressure measurements
HBP is measured by 3G network-equipped automatic sphygmomanometer, based on the cuff-oscillometric principle, which can memorize and transmit systolic/diastolic BP values, heart rate, room temperature and the date and time of each measurement (HEM-7252G-HP; Omron, Japan). Registered patients will be instructed at the clinic how to use the device, then they are asked to take a HBP reading in the sitting position twice every morning within an hour of waking before taking meal or medication, after more than 2 min of rest. Patients are also asked to measure their HBP twice every evening before going to bed. The average of all transmitted HBP measurements during the week before the face-to-face or online visit, is used for the inclusion, randomization, and assessment of reaching BP control. In the control group, self-reported HBP is used to manage blood pressure during the test phase. Office BP is conventionally measured with the HEM 907 (Omron, Japan), a fully automatic device based on the cuff-oscillometric method or HEM-7252G-HP. Blood pressure and heart rate after 2 min of rest in the sitting position are measured twice by physicians at every visit. These devices have been validated previously [Citation20,Citation21] and meet the criteria of the Association for the Advancement of Medical Instrumentation.
Target blood pressure and titration of medication
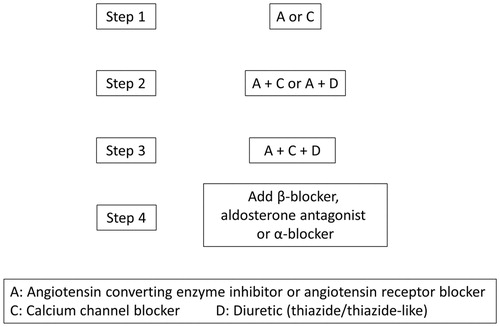
Physicians titrate antihypertensive medication to achieve target HBP of 135/85 mm Hg. The basic principles for antihypertensive treatment is shown in (step 3). Treatment resistance is defined as HBP not controlled on 3 drugs classes given at maximal doses, including a diuretic after 6 months of follow-up. These patients might be referred for further exploration and treatment adjustment at a hypertension center. Medical treatment can consist of all major drug classes. This includes diuretics, α-blockers, β-blockers, calcium-channel blockers (CCBs), angiotensin-converting enzyme inhibitors (ACEIs), angiotensin II type 1 receptor blockers (ARBs), the direct renin inhibitor aliskiren, aldosterone receptor antagonists, centrally acting antihypertensive drugs and vasodilators. Lifestyle changes will be recommended and reinforced during the whole trial, including smoking cessation, moderation of excessive alcoholic intake, control of body weight, and a moderation of dietary salt intake. Patients will be instructed to minimize the exposure to medications affecting blood pressure, including non-steroidal anti-inflammatory drugs, steroids, licorice and decongestants.
Primary and secondary outcomes
The primary endpoint will be time to HBP control in the three randomized groups. Blood pressure control will be defined as a self-measured HBP below 135 mm Hg in systolic and 85 mm Hg in diastolic. Blood pressure control is assumed to be achieved if the aforementioned levels are attained as an average during the week preceding the last office or internet-based visit with at least 5 home measurement sessions. Transmitted HBP measurements will be used for the endpoint decision in all groups.
Secondary endpoints
Secondary endpoints related to blood pressure control (efficacy) are: (i) the proportion of patients reaching blood pressure control on self-measurement at home and office measurement (Blood pressure control on office measurement is a seated blood pressure below 140 mm Hg systolic and 90 mm Hg); (ii) the proportion of patients reaching and maintaining blood pressure control on self-measurement and office measurement at the 6-month follow-up visit; (iii) the intensity of medical treatment; (iv) adverse events, recorded by a self-administered questionnaire; (v) the assessment of drug adherence; (vi) assessment of quality of life; and (vii) analysis of cost-effectiveness.
Sample size
To detect a 1-month difference in time to control of blood pressure, assuming 3 months in the control group and 2 months in the telemonitoring and telemedicine groups, with a two-sided p-value of .05 and 80% power, 148 patients need to be screened per group and 118 randomized, assuming 20% screening failures. In total, 444 patients will be screened for the study.
Selection of patients
The inclusion and exclusion criteria are listed in . Flyer and web-based advertisement will be used for recruitment.
Table 1. Criteria for patient selection in the REMOTE trial.
Follow-up
Patients will be followed up to 6 months after randomization (). Scheduling of next visit will be at the discretion of the physicians, but typically every month. Physicians may recommend earlier visit or telemedicine interviews based on the telemonitored blood pressure and other conditions. Patients will be assessed at every visit or telemedicine interview for reaching the endpoint. Long-term supervised or non-supervised follow-up will continue up to 36 months after the treatment period to evaluate the long-term safety and efficacy of telemonitoring and telemedicine.
Measurements and information to be collected
lists measurements to be obtained at each visit: (i) identification of the center and investigators; (ii) anthropometric characteristics of the patient (e.g. height, weight, hip and waist circumference); (iii) risk factors other than hypertension (e.g. history of diabetes mellitus); (iv) smoking habits and alcohol intake; (v) biochemical measurements required for guiding treatment (e.g. serum creatinine, serum lipid); (vi) information of HBP collected via diary cards during the week preceding the next office visit in case of control group; (vii) telemonitoring reports (all groups but blinded for patients in the control and telemonitoring groups); (viii) information on antihypertensive drug treatment (drug class, timing of administration, and daily dose); (iv) drug treatment other than blood pressure lowering medication (e.g. Lipid-lowering drugs); (x) simple self-administered questionnaires inquiring into side-effects; (xi) the Japanese version of EQ-5D questionnaire for the assessment of quality of life [Citation22]; (xii) the Morisky questionnaire on adherence [Citation23].
Table 2. Measurements and information to be obtained at each visit.
Adherence
Adherence will be monitored by Japanese version of the Morisky questionnaire upon every office and online interview.
Morbidity and mortality
Events in REMOTE will comply with definitions as implemented in previously published randomized clinical trials [Citation24,Citation25]. Adverse events and the incidence fatal and non-fatal events will be recorded.
Quality of life and cost-effectiveness
The quality of life of the patients will be assessed at baseline and at each visit during follow-up, using the Japanese version of EQ-5D questionnaire [Citation22]. Information on any technical difficulties of using the telemonitoring and telemedicine systems will also be collected. Cost-effectiveness will be analyzed from the perspective of the Japanese healthcare system, including both the direct and indirect cost of usual care, telemonitoring and telemedicine.
Data management and statistical analysis
The Department of Endocrinology and Hypertension at Tokyo Women’s Medical University will be in charge of the management of the trial. The stratification and randomization of patients and database management will be assisted by the Studies Coordinating Centre at University of Leuven (SCC). For data management and statistical analysis, the SCC will use SAS software (SAS Institute, Cary, NC, USA). The primary outcomes will be illustrated and analyzed based on Kaplan-Meier estimates. Continuous endpoints related to blood pressure will be analyzed using mixed models.
Discussion
This is the first large-scale intervention trial based on internet-transmitted HBP and telemedicine on the management of hypertension conducted in Japan, and to the best of our knowledge, the first to assess the effect of blood pressure telemonitoring with time to control as the primary outcome. This study targets uncontrolled hypertensive patients without cardiovascular complications. The close-out visit will take place 6 months after randomization, but the patients remain in follow-up for up to 36 months.
Median time required for the control of blood pressure is generally 3 months [Citation26], with a wide range from 8 weeks [Citation27] to 4.5 months [Citation28], depending on research settings and selection of patients. Several clinical trials document the benefit of early blood pressure control on outcome [Citation29–31]. The number of medication changes, and consequently, the length of time to achieve blood pressure control, is shown to be an important indicator of patient adherence during the later phase of treatment [Citation32,Citation33]. With the advent of combination pills, combination therapy as a first-line antihypertensive treatment is sometimes recommended especially for patients with complications [Citation34,Citation35]. However, the rate of combination therapy used as first-line is low, only 3.2–4.2% [Citation35], which may be caused by physicians’ anxiety for side effects such as hypotension. Frequent telemonitoring and telemedicine may enable fast stepping up in treatment and earlier blood pressure control. On the other hand, it is possible that in the telemedicine group, nocebo effects of diminished real-office doctor-patient relationship may negatively affect blood pressure control. This study will clarify these uncertainties.
The strengths of this study include the following: (i) its 3-arm design enabling the exploration on the effects of telemedicine separately from those of telemonitoring; (ii) designation of an independent center for randomization, stratification, and data deposition; (iii) extension of the follow-up beyond 6 months up to 3 years for assessment of long-term effects; and (iv) the inclusion of adherence, quality of life, and economic factors for analyses.
Self-monitoring of biometrics such as HBP and urinary sodium/potassium ratio which reflects dietary salt intake [Citation36], BP telemonitoring, and management by telemedicine are developing themes in the challenge against hypertension. If internet-based HBP telemonitoring with telemedicine without office visits is superior to usual care, this emerging mode of hypertension management could have a significant impact by reducing the number of patients with untreated hypertension, treatment drop-outs, future cardiovascular events, and medical costs, leading to a better quality of life for a large population.
Acknowledgements
We thank Chika Miki for secretarial and Erina Yamaguchi for clerical assistance.
Disclosure statement
The trial is funded by Port, Inc. (Tokyo, Japan) and Omron Corporation (Kyoto, Japan). JY received research grants from Takeda and Tanabe Mitsubishi. Kyotaro Ito and Tomohiro Sonoo work for Port, Inc.
Additional information
Funding
References
- Staessen JA, Kuznetsova T, Stolarz K. Hypertension prevalence and stroke mortality across populations. JAMA. 2003;289:2420–2422.
- Weinehall L, Ohgren B, Persson M, et al. High remaining risk in poorly treated hypertension: the ‘rule of halves’ still exists. J Hypertens. 2002;20:2081–2088.
- Shimamoto K, Ando K, Fujita T, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res. 2014;37:253–390.
- Chobanian AV. Shattuck Lecture. The hypertension paradox-more uncontrolled disease despite improved therapy. N Engl J Med. 2009;361:878–887.
- Bosworth HB, Powers BJ, Olsen MK, et al. Home blood pressure management and improved blood pressure control: results from a randomized controlled trial. Arch Intern Med. 2011;171:1173–1180.
- Kim YN, Shin DG, Park S, et al. Randomized clinical trial to assess the effectiveness of remote patient monitoring and physician care in reducing office blood pressure. Hypertens Res. 2015;38:491–497.
- Burnier M. Medication adherence and persistence as the cornerstone of effective antihypertensive therapy. Am J Hypertens. 2006;19:1190–1196.
- Pickering TG. Home blood pressure monitoring: a new standard method for monitoring hypertension control in treated patients. Nat Clin Pract Cardiovasc Med. 2008;5:762–763.
- Bonafini S, Fava C. Home blood pressure measurements: advantages and disadvantages compared to office and ambulatory monitoring. Blood Press. 2015;24:325–332.
- Asayama K, Ohkubo T, Metoki H, et al. Cardiovascular outcomes in the first trial of antihypertensive therapy guided by self-measured home blood pressure. Hypertens Res. 2012;35:1102–1110.
- McManus RJ, Mant J, Bray EP, et al. Telemonitoring and self-management in the control of hypertension (TASMINH2): a randomised controlled trial. Lancet. 2010;376:163–172.
- McManus RJ, Mant J, Haque MS, et al. Effect of self-monitoring and medication self-titration on systolic blood pressure in hypertensive patients at high risk of cardiovascular disease: the TASMIN-SR randomized clinical trial. JAMA. 2014;312:799–808.
- Asche SE, O’Connor PJ, Dehmer SP, et al. Patient characteristics associated with greater blood pressure control in a randomized trial of home blood pressure telemonitoring and pharmacist management. J Am Soc Hypertens. 2016;10:873–880.
- Pawloski PA, Asche SE, Trower NK, et al. A substudy evaluating treatment intensification on medication adherence among hypertensive patients receiving home blood pressure telemonitoring and pharmacist management. J Clin Pharm Ther. 2016;41:493–498.
- Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA. 2013;310:46–56.
- Lee CJ, Park S. The role of home blood pressure telemonitoring for blood pressure control. Pulse (Basel). 2016;4:78–84.
- Omboni S, Gazzola T, Carabelli G, et al. Clinical usefulness and cost effectiveness of home blood pressure telemonitoring: meta-analysis of randomized controlled studies. J Hypertens. 2013;31:455–468.
- Tani S, Asayama K, Oiwa K, et al. The effects of increasing calcium channel blocker dose vs. adding a diuretic to treatment regimens for patients with uncontrolled hypertension. Hypertens Res. 2017;40:892–898.
- Kario K, Tomitani N, Kanegae H, et al. Comparative effects of an angiotensin ii receptor blocker (ARB)/diuretic vs. ARB/calcium-channel blocker combination on uncontrolled nocturnal hypertension evaluated by information and communication technology-based nocturnal home blood pressure monitoring: the NOCTURNE study. Circ J. 2017;81:948–957.
- El Assaad MA, Topouchian JA, Darne BM, et al. Validation of the Omron HEM-907 device for blood pressure measurement. Blood Press Monit. 2002;7:237–241.
- Takahashi H, Yoshika M, Yokoi T. Validation of two automatic devices: omron HEM-7252G-HP and Omron HEM-7251G for self-measurement of blood pressure according to the European Society of Hypertension International Protocol revision 2010. Blood Press Monit. 2015;20:286–290.
- Shiroiwa T, Fukuda T, Ikeda S, et al. Japanese population norms for preference-based measures: EQ-5D-3L, EQ-5D-5L, and SF-6D. Qual Life Res. 2016;25:707–719.
- Morisky DE, Ang A, Krousel-Wood M, et al. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertension. 2008;10:348–354.
- Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–1898.
- Haller H, Ito S, Izzo JL Jr, et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med. 2011;364:907–917.
- Hong SH, Wang J, Tak S. A patient-centric goal in time to blood pressure control from drug therapy initiation. Clinical and Translational Science. 2013;6:7–12.
- Weir MR, Levy D, Crikelair N, et al. Time to achieve blood-pressure goal: influence of dose of valsartan monotherapy and valsartan and hydrochlorothiazide combination therapy. Am J Hypertens. 2007;20:807–815.
- Kerfoot BP, Turchin A, Breydo E, et al. An online spaced-education game among clinicians improves their patients’ time to blood pressure control: a randomized controlled trial. Circ Cardiovasc Qual Outcomes. 2014;7:468–474.
- Weber MA, Julius S, Kjeldsen SE, et al. Blood pressure dependent and independent effects of antihypertensive treatment on clinical events in the VALUE Trial. Lancet. 2004;363:2049–2051.
- Staessen JA, Thijisq L, Fagard R, et al. Effects of immediate versus delayed antihypertensive therapy on outcome in the Systolic Hypertension in Europe Trial. J Hypertens. 2004;22:847–857.
- Basile JN, Chrysant S. The importance of early antihypertensive efficacy: the role of angiotensin II receptor blocker therapy. J Hum Hypertens. 2006;20:169–175.
- Caro JJ, Speckman JL, Salas M, et al. Effect of initial drug choice on persistence with antihypertensive therapy: the importance of actual practice data. CMAJ. 1999;160:41–46.
- Neutel JM, Smith DH. Improving patient compliance: a major goal in the management of hypertension. J Clin Hypertens (Greenwich). 2003;5:127–132.
- Nash DT, Crikelair N, Zappe D. Achieving BP goals with valsartan and HCTZ alone and in combination: pooled analysis of two randomized, double-blind, placebo-controlled studies. Curr Med Res Opin. 2008;24:2617–2626.
- Weir S, Juhasz A, Puelles J, et al. Relationship between initial therapy and blood pressure control for high-risk hypertension patients in the UK: a retrospective cohort study from the THIN general practice database. BMJ Open. 2017;7:e015527.
- Yatabe MS, Iwahori T, Watanabe A, et al. Urinary sodium-to-potassium ratio tracks the changes in salt intake during an experimental feeding study using standardized low-salt and high-salt meals among healthy Japanese volunteers. Nutrients. 2017;9:E951.