Abstract
Purpose: Population-based studies estimating prevalence’s of white-coat, masked and sustained hypertension in non-European adolescents are needed, particularly in developing countries. Aiming to determine these estimates and, additionally identify factors associated to these conditions this study was conducted.
Materials and methods: Cross-sectional study with a representative sample of secondary school students from a Brazilian state capital. Office measurements were performed with validated semi-automatic devices. Home BP (blood pressure) monitoring protocol included two day-time and two evening-time measurements over 6 days. Adolescents’ were classified as: normotensives (office and home BP <95th percentile); sustained hypertensives (office and home BP ≥95th percentile); white-coat hypertensives (office BP ≥95th percentile and home BP <95th percentile) and masked hypertensives (office BP <95th percentile and home BP ≥95th percentile). Logistic regression models were built to identify if sex, age, BMI and family history of HTN were independently associated with white-coat, masked and sustained hypertension.
Results: In a sample of 1024 adolescents, prevalence of white-coat, masked and sustained hypertension was 7.5%, 2.2% and 1.7%, respectively. Male sex was positively associated with white-coat hypertension (OR 2.68; 95%CI 1.58–4.54; p < 0.001). BMI was positively associated with both white-coat (OR 1.23; 95%CI 1.16–1.30; p < 0.001) and sustained hypertension (OR 1.19; 95%CI 1.11–1.29; p < 0.001). None of the independent variables were associated with masked hypertension in this population.
Conclusion: The estimated prevalence of white-coat hypertension, masked and sustained hypertension in a population of non-European adolescents assessed by home BP monitoring was 7.5%, 2.2% and 1.7% respectively. Male sex was positively associated with white-coat hypertension in these adolescents while BMI was positively associated with both white-coat and sustained hypertension.
Introduction
Hypertension (HTN) prevalence in children and adolescents is increasing, which has been mainly attributed to the obesity epidemic [Citation1]. This situation turned paediatric HTN into a significant public health issue and driven a considerable amount of research in the last two decades [Citation2].
Conventional office blood pressure measurements performed by a healthcare provider were the basis for hypertension diagnosis in children and adolescents until recently [Citation3]. However, as in adults, office BP in these age groups exhibits a number of limitations: the technique itself, the involvement of an observer, the statistical issue of regression to the mean, and the white-coat and masked hypertension phenomena [Citation3]. Therefore, office BP measurements should be used as reference for BP values for children and adolescents, and values obtained out-of-office used to improve the evaluation of untreated and treated individuals [Citation4]. Several studies have demonstrated the value of ambulatory blood pressure monitoring (ABPM) in paediatric hypertension, whereas home BP monitoring has been evaluated more recently [Citation5].
The assessment of out-of-office blood pressure (BP) using either ABPM or home BP monitoring has allowed the detection of white-coat, masked and sustained hypertension [Citation6]. With the exception of sustained hypertension, in the other two conditions office and out-of-office BP values do not agree, depending on the setting [Citation7]. In the paediatric population, white-coat HTN is associated with hypertensive end-organ damage and exaggerated response to treadmill exercise [Citation8], while masked HTN is associated with persistent hypertension and increased cardiovascular risk later in life [Citation9].
Despite the clinical relevance of white-coat, masked and sustained hypertension, to our knowledge, only one European study assessed the prevalence of these conditions in children and adolescents using home BP monitoring. Even more concerning is the absence of studies in developing countries addressing this matter.
Due to this limited data, we conducted a population-based study using office and home BP measurements in a non-European population from a developing country. Our objective was to estimate the prevalence of white-coat, masked and sustained hypertension, as well as associated factors, in adolescents using home BP monitoring as the out-of-office blood pressure measurement technique.
Methods
A school-based cross-sectional study was performed with secondary school students (ages between 12 and 17 years) from both public and private schools. The study took place in Goiânia, a city located in the Midwest region of Brazil, with a population of 1,302,001 inhabitants at enrollment period [Citation10].
The research protocol was approved by the Federal University of Goiás’ Research Ethics Committee. Eligible adolescents who agreed to participate were asked to sign an informed consent form, and so were their guardians.
Sample size calculation considered: a population of 133,528 students enrolled both in public and private schools of Goiânia [Citation10], masked hypertension prevalence of 7% [Citation11], an absolute error of 2%, and a confidence level of 95%. These parameters determined a sample size of 1,024 students and a total of 1,030 were enrolled. Considering the prevalence of hypertension among Brazilian adolescents of 8.12% [Citation12] and using a 95% confidence interval (CI), this sample size allows estimate the prevalence of hypertension with a 1.7% margin of error.
A total of 132 public and private schools from the 9 regions of the city were identified, with a ratio of 2 public schools for each private one. Following this initial mapping, 6 public and 3 private schools from each region were drawn and invited to participate. Each school was contacted following the drawing order. If a school did not agree to participate, replacement was made by the same school type (public replaced by public and private replaced by private). At the end 26 schools agreed to participate (17 public and 9 private). Adolescents’ enrolled in these institutions were selected by random drawing, stratified by age and gender.
The exclusion criteria were: physical disabilities that prevented the practice of physical activities (PA), pregnancy, disabling chronic diseases and continuous use of medication.
Data was collected by a trained team over 13 months. The team consisted of four supervisors (with extensive experience on using the blood pressure monitoring devices) and nine data collectors (all of them college students from Physical Therapy or Nutrition programs).
A standardized questionnaire was used for data collection and included: identification (name, date of birth, age, gender, self-reported ethnicity – white or non-white); school type (public or private); personal history (current diseases and medications); family history of hypertension, diabetes, obesity and cardiovascular disease (history in parents or grandparents was considered); tobacco use; alcohol consumption; and physical activity - the International Physical Activity Questionnaire (IPAQ) was used [Citation13]. Anthropometric measurements (height, weight, arms circumference and waist circumference) were also performed.
Office BP measurements were taken based on the 4th Task Force recommendations on cuff size and adolescents’ preparation for measurement [Citation14]. A device previously validated to be used in adolescents was used (OMRON semi-automatic equipment, model HEM-705CP) [Citation15]. Three different cuff sizes (9 × 16 cm, 13 × 23 cm and 15 × 30 cm) were selected according to the adolescent’s arm circumference (80 to 100%). All measurements were performed at proper schools offices, in two different moments (one-week apart). At each time point, two measurements were obtained (with a three-minute interval), reaching a total of four BP readings. Mean values of second readings on each time-point (one week apart) were chosen to be used for data analyses, since it has been previously shown that these values had a better correlation with out-of-office BP measurements in this population [Citation4].
Home BP monitoring protocol included two readings in the morning (between 6 a.m. and 10 a.m.) ant two in the evening (between 6 p.m. and 10 p.m.), for six consecutive days, with a total of 24 readings [Citation16]. All measurements should be performed in sitting position, after 5 minutes of rest, using the same arm, equipment, and cuffs sizes used for office BP measurements to avoid systematic differences. Exams were considered valid with at least 12 measurements (50%) performed according to standardized protocols [Citation17]. Home BP monitoring devices and specific home BP forms were given to adolescents, who were trained on how to use it and fill in the form. Training sessions were conducted by data collection supervisors and included a theoretical presentation followed by a practical session, in which adolescents had the opportunity to test the equipment and clarify all doubts. Home measurements saved in equipment memories were printed and compared to those reported in home BP forms. When there was a discrepancy between measures or when adolescents reported that other person used the equipment, the exam was excluded. Mean values of all home BP monitoring, excluding the first day, were considered for analyses.
Adolescents were classified according to the available normalcy tables for office [Citation14] and home BP monitoring [Citation18] as: normotensives (office and home BP <95th percentile); sustained hypertensives (office and home BP ≥95th percentile); white-coat hypertensives (office BP ≥95th percentile and home BP <95th percentile) and masked hypertensives (office BP <95th percentile and home BP ≥95th percentile).
Nutritional status was classified as: normal weight, overweight and obesity according to body mass index (BMI - body mass in kilograms divided by the square of the body height in meters). Reference curves from the World Health Organization (WHO) [Citation19] were adopted, using the BMI-for-age chart, according to sex.
The short-form (Version 8) of the IPAQ was applied to estimate the prevalence of sedentary lifestyle, considering the PA practiced in the previous week as a reference [Citation13]. The questions addressed the frequencies and durations of light (walking), moderate and vigorous PA. Sedentary lifestyle was defined as less than 300 minutes of moderate or vigorous physical activity per week [Citation20].
Regarding alcohol consumption adolescents were asked if they had any alcohol consumption in the last 30 days and the positive answers were defined as alcohol consumption. Smoking was defined by self-report of having smoked at least one day over the previous 30 days [Citation21].
The variables were analysed using the software Stata 14.0 (StataCorp., College Station, Texas, USA). The Shapiro-Wilkins test was used and confirmed that the continuous variables were normally distributed. Descriptive population analyses using mean values and standard deviations of continuous variables and absolute number and proportions of dichotomous ones were performed. The continuous variables were compared with ANOVA and the dichotomous with Chi-square Test. Multivariate logistic regression models were built to identify variables independently associated with white-coat, masked and sustained hypertension. The independent variables were sex, age, BMI and family history of HTN and the models were adjusted for sedentary lifestyle, alcohol consumption, smoking and ethnicity. A significance level was set as 5%.
Results
A total of 1,030 adolescents were enrolled in the study and 1,024 (99.4%) were included in this assessment. The reasons for the 6 exclusions were: discrepancy between home BP measurements and home BP form reports (2); equipment used by someone other than the adolescent (1); less than 12 home BP measurements performed (3). All home BP measurements were taken by the adolescents as oppositely to measurements taken by relatives or parents. None of the adolescents reported themselves as hypertensives in their personal history assessments.
Prevalence of white-coat hypertension assessed by office and home BP was higher than the prevalence of masked and sustained hypertension. The blood pressure classification considering office and home blood pressure in these adolescents is shown in .
Figure 1. Blood pressure classification considering office and home blood pressure in adolescents, Goiania - Brazil (n = 1024).
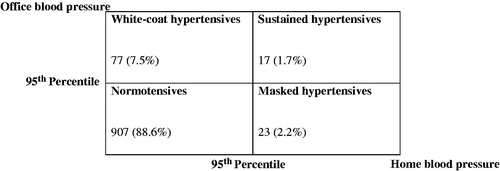
The proportion of male adolescents was higher among all the hypertensive classes (white-coat, masked and sustained hypertension) and lower in the normotensive adolescents. The higher mean BMIs were found in the sustained and white-coat hypertensives while the lowest in the normotensives. This same pattern was seen in WC mean values. The population characteristics assessed according to blood pressure classification are reported in .
Table 1. Population characteristics according to blood pressure classification (n = 1024), Goiania - Brazil.
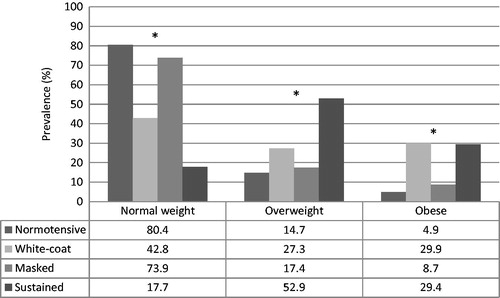
Adolescents with normal weight were the majority among the normotensives and those with masked hypertension. Oppositely overweight and obesity were more frequent among white-coat and sustained hypertensive adolescents. The nutritional status distribution among the different blood pressure classifications is shown in .
Figure 2. Adolescent’s nutritional status distribution according to blood pressure classification (n = 1024). Goiania - Brazil.
* Nutritional status distribution difference within blood pressure classification - Statistically significant at α = 0.05.
Male sex was independently and positively associated with white-coat but not masked or sustained hypertension in these adolescents. BMI was positively associated with white-coat and sustained hypertension. None of the independent variables included in the model were associated with masked hypertension in this population. shows the variables independently associated with white-coat, masked and sustained hypertension.
Table 2. Variables independently associated to white-coat, masked and sustained hypertension, Goiania - Brazil.
Discussion
A representative sample of more than 1000 adolescents from a large Brazilian city with appropriate office and home BP readings was assessed. This is the first population-based study conducted with non-European adolescents from a developing country estimating the prevalence of different blood pressure classifications using home BP monitoring. White-coat, masked and sustained hypertension prevalence’s of 7.5%, 2.2% and 1.7%, respectively, were found in our sample. Additionally, male sex was positively associated with white-coat hypertension while BMI was positively associated with both white-coat and sustained hypertension.
White-coat HTN frequency in children and adolescents ranges from 1 to 44% and this wide variation is probably a result of the criteria used to establish diagnosis. Masked HTN occurs in approximately 10% of children and adolescents [Citation22]. Most studies estimating these prevalences were conducted using ABPM. There is only one population-based study, which was conducted in Europe that used home BP monitoring in the paediatric population to assess white-coat, masked and sustained hypertension. It was published by Stergiou et al. (2009) [Citation6] and one of the main differences from our study is that both children and adolescents were included (mean age of 12.3 ± 3.3 years), and not only adolescents (mean age 14.7 ± 1.6years) as we did. This study reported a prevalence of white-coat, masked and sustained hypertension using the mean BP value of two office visits of 2.1%, 4.2% and 1.8% respectively. We found a higher prevalence of white-coat hypertension (7.5%), lower of masked hypertension (2.2%) and roughly the same prevalence of sustained hypertension (1.7%). These differences may be explained by the use of same reference values for office BP in both studies, despite the differences in the sample characteristics (non-European × European).
The higher frequency of overweight and obese individuals among hypertensive children and adolescents is frequently reported in the literature [Citation2,Citation6,Citation23] and this pattern is usually seen in white-coat and sustained hypertensives but not in masked hypertensives [Citation6,Citation24]. In our study, we could confirm this pattern of distribution. It is interesting to note that mean BMI in normotensive and masked hypertensives was similar in our study (20.31 ± 3.35 × 21.29 ± 3.75 kg/m2 – p = 0.167). The same similarity was found in the nutritional status distribution between these two groups (80.4 × 73.9% - p = .439 for normal weight; 14.8 × 17.4% - p = .729 for overweight and 4.9 × 8.7% - p = .409 for obese).
Male sex was independently and positively associated with white-coat hypertension. This association, which was not found for masked or sustained HTN, has not been shown previously in the literature when addressing children and adolescents. In adults, oppositely, white-coat hypertension is associated with female sex [Citation25]. Further investigations are needed to help clarifying this matter.
BMI was positively associated with white-coat and sustained hypertension. The association of increasing body weight and hypertension in adolescents has been reported in the literature [Citation2] but not addressing specifically white-coat and sustained hypertension. None of the independent variables included in the model were associated with masked hypertension in this population.
Since it is clearly not practical to perform out-of-office BP monitoring in all adolescents with normal office BP in order to unmask those with ambulatory HTN, identify those at high risk is imperative. Our results show that BMI and nutritional status distribution should not be used with this purpose since both variables were similar in masked hypertensives adolescents and normotensives ones. Additionally, BMI was not associated with masked hypertension in our sample. Therefore, the hypothesis that masked hypertension is an intermediate phenotype of sustained hypertension [Citation6] is not confirmed with our data.
The reference values for home BP monitoring [Citation18] that were used to identify home hypertension in this study were obtained from the same population. These recently published values are the only available diagnostic thresholds for home BP monitoring in non-European adolescents. Further studies using these reference values can be compared to our results.
One aspect of home BP monitoring in adolescents that was clearly shown in our study is the feasibility of the method. We used a protocol that required 6 consecutive days of measurements and yet 99.4% of our sample provided a valid result. This feasibility has been shown in different ethnic populations [Citation26–28] and is now confirmed in a large scale population-based study in a developing country.
A strength of this study is the use of a properly validated automated oscillometric device for home BP monitoring in adolescents [Citation15]. Considering that several validation studies conducted with children and adolescents did not fulfill all the requirements of validation protocols [Citation29], selecting the appropriate device is a priority in this kind of epidemiological studies. Another strength is the inclusion of adolescents from both public and private schools, since there is a big difference in ethnicity and parents’ income between the two types of school in Brazil (higher number of white students and from wealthier socioeconomic classes in private schools) [Citation30].
A limitation of this study is the fact that even though our sample is representative of adolescents living in Goiania, it does not have a nationwide representativeness. Despite that, when we compare the prevalence of hypertension in our study using just the office measurements as reference (white-coat and sustained hypertension – 9.2%) with the prevalence of hypertension in a national representative study recently published (roughly 10%) [Citation23] the results are similar. The same similarity is observed in the comparison with a meta-analysis published in 2013 that assessed HTN prevalence among Brazilian adolescents (8.1%) [Citation12].
Using international reference values for office BP, and not Brazilian ones, is another potential limitation. It is justified by the absence of national reference values for office BP measurements in adolescents. Additionally, these reference values have been extensively used in studies estimating the prevalence of hypertension all over the world [Citation12,Citation23,Citation31–34] and are recommended by the 2016 European Society of Hypertension Guidelines for the Management of High Blood Pressure in Children and Adolescents [Citation2] and the by the 7th Brazilian Guideline of Arterial Hypertension in its chapter addressing hypertension in children and adolescents [Citation35].
In conclusion, the estimated prevalence of white-coat hypertension, masked and sustained hypertension in adolescents assessed by home BP monitoring was 7.5%, 2.2% and 1.7% respectively. Male sex was positively associated with white-coat hypertension in these adolescents while BMI was positively associated with both white-coat and sustained hypertension.
Other internationals population-based publications are required to help understanding not only regional differences in the prevalence of white-coat, masked and sustained hypertension in adolescents, but also the factors associated to such clinically relevant conditions.
Acknowledgments
The project was funded by the Brazilian National Council for Scientific and Technological Development (CNPq).
Disclosure statement
The project was funded by the Brazilian National Council for Scientific and Technological Development (CNPq).
No potential, perceived, or real conflict of interest needs to be disclosed. The study sponsor was not involved in the study design; collection, analysis, and interpretation of data; writing of the report; and the decision to submit the manuscript for publication.
Additional information
Funding
References
- Muntner P, He J, Cutler JA, et al. Trends in blood pressure among children and adolescents. Jama 2004;291:2107–2113.
- Lurbe E, Agabiti-Rosei E, Cruickshank JK, et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. 2016;34:1887–1920.
- Kollias A, Dafni M, Poulidakis E, et al. Out-of-office blood pressure and target organ damage in children and adolescents: a systematic review and meta-analysis. J Hypertens. 2014;32:2315–2331. discussion 2331.
- Jardim TV, Gaziano TA, Nascente FM, et al. Office blood pressure measurements with oscillometric devices in adolescents: a comparison with home blood pressure. Blood Press. 2017;1–7. doi: 10.1080/08037051.2017.1312279
- Stergiou GS, Alamara CV, Salgami EV, et al. Reproducibility of home and ambulatory blood pressure in children and adolescents. Blood Press Monit. 2005;10:143–147.
- Stergiou GS, Rarra VC, Yiannes NG. Prevalence and predictors of masked hypertension detected by home blood pressure monitoring in children and adolescents: the Arsakeion School study. Am J Hypertens. 2009;22:520–524.
- Lurbe E, Ingelfinger JR. Blood pressure in children and adolescents: current insights. J Hypertens. 2016;34:176–183.
- Kavey RE, Kveselis DA, Atallah N, et al. White coat hypertension in childhood: evidence for end-organ effect. J Pediatr. 2007;150:491–497.
- Lurbe E, Torro I, Alvarez V, et al. Prevalence, persistence, and clinical significance of masked hypertension in youth. Hypertension. 2005;45:493–498.
- Brazil Demographic Census 2010. In: (IBGE). BIoGaS, editor. Rio de Janeiro, Brazil: Brazilian Institute of Geography and Statistics (IBGE); 2012.
- Verberk WJ, Kessels AG, de Leeuw PW. Prevalence, causes, and consequences of masked hypertension: a meta-analysis. Am J Hypertens. 2008;21:969–975.
- Magliano ES, Guedes LG, Coutinho ES, et al. Prevalence of arterial hypertension among Brazilian adolescents: systematic review and meta-analysis. BMC Public Health. 2013;13:833.
- Guedes DP, Lopes CC, Guedes JERP. Reprodutibilidade e validade do Questionário Internacional de Atividade Física em adolescentes. Rev Bras Med Esporte. 2005;11:151–158.
- The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576.
- Stergiou GS, Yiannes NG, Rarra VC. Validation of the Omron 705 IT oscillometric device for home blood pressure measurement in children and adolescents: the Arsakion School Study. Blood Press Monit. 2006;11:229–234.
- [V Guidelines for ambulatory blood pressure monitoring (ABPM) and III Guidelines for home blood pressure monitoring (HBPM)]. Arq Bras Cardiol. 2011;97:1–24.
- Mallick S, Kanthety R, Rahman M. Home blood pressure monitoring in clinical practice: a review. Am J Med. 2009;122:803–810.
- Jardim TV, de Souza Carneiro C, Morais P, et al. Home blood pressure normalcy in non-European adolescents. J Hypertens. 2018;36:61–68.
- de Onis M, Onyango AW, Borghi E, et al. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85:660–667.
- Hallal PC, Andersen LB, Bull FC, et al. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet (London, England). 2012;380:247–257.
- Warren CW, Jones NR, Peruga A, et al. Global youth tobacco surveillance, 2000-2007. Morbidity and Mortality Weekly Report Surveillance Summaries (Washington, DC: 2002) 2008;57:1–28.
- Lurbe E, Torro MI, Alvarez J. Ambulatory blood pressure monitoring in children and adolescents: coming of age? Curr Hypertens Rep. 2013;15:143–149.
- Bloch KV, Klein CH, Szklo M, et al. ERICA: prevalences of hypertension and obesity in Brazilian adolescents. Revista De Saúde Pública 2016;50:9s.
- Stabouli S, Kotsis V, Toumanidis S, et al. White-coat and masked hypertension in children: association with target-organ damage. Pediatr Nephrol. 2005;20:1151–1155.
- Sheppard JP, Fletcher B, Gill P, et al. Predictors of the Home-Clinic Blood Pressure Difference: A Systematic Review and Meta-Analysis. Am J Hypertens. 2016;29:614–625.
- Stergiou GS, Yiannes NG, Rarra VC, et al. Home blood pressure normalcy in children and adolescents: the Arsakeion School study. J Hypertens. 2007;25:1375–1379.
- Salgado CM, Jardim PCBV, Viana JKB, et al. Home blood pressure in children and adolescents: a comparison with office and ambulatory blood pressure measurements. Acta Paediatr. 2011;100:e163–e168.
- Asayama K, Staessen JA, Hayashi K, et al. Mother-offspring aggregation in home versus conventional blood pressure in the Tohoku Study of Child Development (TSCD). Acta Cardiol. 2012;67:449–456.
- Stergiou GS, Boubouchairopoulou N, Kollias A. Accuracy of Automated Blood Pressure Measurement in Children: Evidence, Issues, and Perspectives. Hypertension. 2017;69:1000
- Nascente FMN, Jardim TV, Peixoto MRG, et al. Sedentary lifestyle and its associated factors among adolescents from public and private schools of a Brazilian state capital. BMC Public Health. 2016;16:1177.
- Bozza R, Campos W, Barbosa Filho VC, et al. High Blood Pressure in Adolescents of Curitiba: Prevalence and Associated Factors. Arq Bras Cardiol. 2016;106:411–418.
- Chiolero A, Cachat F, Burnier M, et al. Prevalence of hypertension in schoolchildren based on repeated measurements and association with overweight. J Hypertens. 2007;25:2209–2217.
- Katona E, Zrinyi M, Lengyel S, et al. The prevalence of adolescent hypertension in Hungary - the Debrecen hypertension study. Blood Press. 2011;20:134–139.
- Maldonado J, Pereira T, Fernandes R, et al. An approach of hypertension prevalence in a sample of 5381 Portuguese children and adolescents. The AVELEIRA registry. “Hypertension in children”. Blood Press. 2011;20:153–157.
- Malachias MVB, Koch V, Colombo C, et al. 7th Brazilian Guideline of Arterial Hypertension: Chapter 10 - Hypertension in Children and Adolescents. Arq Bras Cardiol 2016;107:53–63.
