Abstract
Objectives: Cerebral white matter lesions (WMLs) are regarded to be subclinical ischemic changes of the cerebral parenchyma. Many previous studies have shown that baseline blood pressure (BP) is one of the most important factors for WMLs, but the relation between exercise BP and WMLs has not been fully evaluated. So, we sought to investigate the relationships between cerebral WMLs and peak exercise BP.
Methods: Brain magnetic resonance imaging scan and treadmill testing were performed simultaneously in 130 consecutive subjects without history of stroke or transient ischemic stroke.
Results: Among 130 subjects, 42 individuals (32%) presented WMLs. Individuals with WMLs were older than those without WMLs, and baseline systolic BP and pulse pressure were higher in subjects with WMLs. During treadmill test, peak exercise systolic BP was more significantly elevated in subjects with WMLs. In multivariable logistic regression analysis, elevated baseline systolic BP, not peak exercise systolic BP, was associated with the presence of WMLs, independently of age. However, in multivariable logistic regression analysis of 88 normotensive subjects, elevated peak systolic BP during exercise was the only determinant for the presence of WMLs.
Conclusions: Elevated peak systolic BP during exercise is significantly related with WMLs, subclinical small vessel disease of brain, especially in normotensive subjects.
Introduction
Cerebral white matter lesions (WMLs) are frequently seen on T2 weighted magnetic resonance imaging (MRI) scans in elderly people, especially in those with hypertension [Citation1,Citation2]. These lesions are regarded to be subclinical ischemic changes of the cerebral parenchyma and associated with greater risk of stroke, depression, cognitive dysfunction and dementia [Citation3–5]. Although the exact pathogenesis of WMLs is not yet clear, they are regarded to be the consequence of arteriosclerosis, cerebral hypoperfusion or ischemia [Citation6,Citation7]. Many previous studies have shown that hypertension is one of the most important factors for the presence of WMLs [Citation8–10]. Uncontrolled hypertension leads to the increase of WMLs and adequate antihypertensive treatment could reduce their progression [Citation11,Citation12]. However, some studies demonstrated inconsistent relationship of resting blood pressure (BP) with WMLs, reporting that resting BP was not associated with WMLs and BP control could not reduce the progression of WMLs [Citation13,Citation14].
An exercise BP response is associated with various cardiovascular risk factors and adverse cardiovascular outcomes [Citation15–17]. Some recent studies involving long-term follow-up in healthy subjects showed the association between elevated exercise BP and cardiovascular outcomes [Citation18,Citation19]. Even without apparent hypertension, elevated exercise BP could result in target organ damage [Citation20,Citation21]. Although the brain could be the primary target organ of high BP, the relation between exercise BP and WMLs has not been fully evaluated. Many previous studies only evaluated the association of resting BP and cerebral WMLs [Citation8–10]. We hypothesized that exercise BP would be associated with cerebral WMLs independently of baseline BP. So, we investigated the relationships of the presence of cerebral WMLs with peak exercise BP.
Methods
Participants
We recruited 130 individuals consecutively without a history of stroke or transient ischemic stroke, who visited Chaum Medical Center from December 2012 to October 2015 for routine health check-up. Patients with history of atrial fibrillation, cardiovascular disease or chronic disease such as renal failure, pulmonary disease and liver disease were excluded from this study. The subjects underwent both brain MRI and treadmill exercise test. At the time of enrollment, all medical histories were reviewed by a physician and complete physical examination and laboratory assessment were completed. This study was approved by our institutional ethics committee and was in compliance with the Declaration of Helsinki. All study participants provided written informed consent.
Baseline BP measurement
Baseline brachial BP was measured with a mercury sphygmomanometer after at least 5 minutes of rest in the sitting position during two different visits to our outpatient clinic. The average of three measurements was used as the representative value.
Exercise testing
Resting systolic and diastolic BPs were measured in the sitting position by an automated BP monitor (Tango Stress BP, Morisville NC, USA) after at least 5 minutes of rest. Symptom-limited exercise testing was performed according to the Bruce protocol on a GE treadmill (T-2100 treadmill, GE Healthcare, Waukesha, USA). A 12-lead electrocardiogram was also recorded throughout the test. Exercise BP was measured during the last minute of each 3 minute stage and at peak exercise using an automated BP monitor (Tango Stress BP, Morrisville NC, USA) with the arm relaxed at the patient’s side without holding on to the side bar of the treadmill. The exercise test was stopped if the participants had more than 90% of the predicted maximal heart rate and a systolic BP >240 mmHg or a decrease in systolic BP ≥10 mmHg. Exercise was also terminated when subjects were too fatigued to continue or when ischemic ST-segment changes were observed [Citation22].
Brain MRI and diagnosis of WMLs
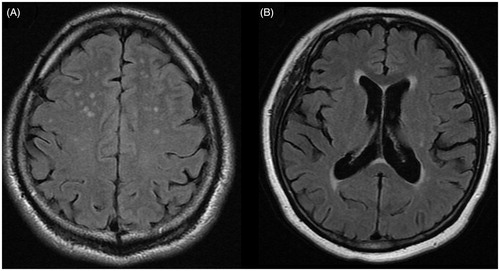
MRI examinations were performed with 1.5 T MRI system (Optima MR450w, GE Healthcare, Milwaukee, WI). The standardized MRI protocols were applied to obtain axial T1 and T2-weighted and FLAIR scans with a slice thickness of 5 mm (T2 flair echo time, 130 ms; repetition time, 6000 ms; inversion time, 2200 ms; echo train length, 19; flip angle, 90 degrees; number of average, 2). The MRI images were evaluated by an experienced radiologist who was blinded to all clinical information. WMLs were defined as areas of brain parenchyma with an increased signal on both T2-weighted and FLAIR images without corresponding hypointense lesions on the T1 weighted scans (). The severity of deep white matter hyperintensity was graded using Fazekas scale (absent, grade 0; punctuate, grade 1; early confluent, grade 2; confluent, grade 3). The severity of periventricular white matter hyperintensity was graded as follows using Fazekas scale; 0 = absent, 1 = caps or pencil-thin lining, 2 = smooth halo, 3 = irregular extending into deep white matter [Citation23].
Statistical analyses
Continuous variables are presented as the mean ± SD and categorical variables as the number of subjects (percentage). The hypothesis of normality was assessed using the Shapiro-Wilk test. Differences in continuous variables were evaluated using t-tests for independent samples or Mann-Whitney U tests. Chi-square tests or Fisher’s exact tests were performed for testing differences in categorical variables. The risk factors for WMLs were evaluated by using univariate and multiple logistic regression analyses. Briefly, variables that were significant at p < .10 level based on the univariate logistic regression analysis were included in an initial multiple logistic regression model. Then multivariate analysis was performed using multiple logistic regression with a stepwise selection procedure. Age and gender were adjusted irrespective of their associations. All statistical analyses were performed using R language ver. 3.01 (R Foundation for Statistical Computing, Vienna, Austria). A p value <.05 was considered to be statistically significant.
Results
One hundred and thirty individuals (mean age, 52 years; 86 men) underwent exercise treadmill test and brain MRI scan. Among these subjects, 29 (22%) had a history of hypertension, 9 (7%) had diabetes mellitus and 16 (12%) had hyperlipidemia. Forty-two individuals (32%) presented WMLs on brain MRI. Among them, 40 individuals showed deep white matter hyperintensities; 32 subjects showed grade1 WMLs and 8 showed grade 2 WMLs. In 12 subjects with deep white matter hyperintensities, periventricular white matter hyperintensities were also identified. Two subjects showed only periventricular white matter hyperintensities. All the periventricular WMLs were grade 1. Due to the small number of subjects, grade 1 and grade 2 WMLs were analyzed together to identify the related factors for the presence of WMLs.
There were no significant differences in clinical or laboratory characteristics between the participants with and without WMLs, with the exception of age, history of hypertension, baseline systolic BP and pulse pressure (). Subjects with WMLs were older than those without WMLs (58 ± 8 vs 49 ± 10 years, p < .01). Baseline systolic BP (132 ± 10 vs 121 ± 14 mmHg, p < .01) and pulse pressure (50 ± 7 vs 46 ± 6 mmHg p = .01) were higher in the subjects with WMLs.
Table 1. Clinical, laboratory and treadmill exercise data for subjects with and without WMLs.
The results of the exercise treadmill test were shown in . During the exercise, peak exercise systolic BP and pulse pressure were significantly elevated in the subjects with WMLs. The difference of the resting and exercise BP during treadmill test increased more significantly in the individuals with WMLs.
Age, baseline systolic BP, baseline pulse pressure, peak exercise systolic BP, peak exercise pulse pressure, and the difference between the resting and exercise systolic BP were associated with the presence of WMLs in univariate logistic regression analysis. However, after adjusting for potentially confounding variables in multivariate logistic regression analysis, elevated baseline systolic BP was found to be the risk factor for the presence of WMLs independently of age ().
Table 2. Logistic regression analysis of potential risk factors for WMLs.
Among 88 normotensive individuals with normal range of baseline BP without history of hypertension, 25 subjects (28%) showed WMLs on brain scan. Even in normotensive subjects, individuals with WMLs were older than those without WMLs and showed higher baseline systolic BP. During exercise, peak systolic BP, pulse pressure, and the difference between the resting and exercise BP were also significantly elevated in individuals with WMLs (). In univariate logistic regression analysis, age, baseline systolic BP, peak exercise systolic BP, peak exercise pulse pressure, and the difference between the resting and exercise BP were associated with the presence of WMLs. However, after adjusting for potentially confounding variables in multivariate logistic regression analysis for normotensive subjects, elevated peak exercise systolic BP was the main determinant of the presence of WMLs ().
Table 3. Clinical, laboratory and treadmill exercise data for normotensive subjects with and without WMLs.
Table 4. Logistic regression analysis of potential risk factors for WMLs in normotensive individuals.
Discussion
In this study, we sought to evaluate whether elevated BP during exercise could induce target organ damage such as small vessel disease of brain. The principal finding of our study was that elevated systolic BP during exercise is significantly associated with WMLs especially in normotensive subjects. Baseline systolic BP was related with WMLs as demonstrated in previous studies. However, in only normotensive subjects, elevated systolic BP at peak exercise, was the main determinant for the presence of WMLs.
The white matter of the brain is composed of nerve fibers, neuroglial cells, vessels and interstitial space. WMLs on brain MRI represents various neuropathological findings such as loss of parenchyma, vascular changes, atrophy and necrosis with scarring [Citation24]. These lesions are regarded to be associated with greater risk of stroke, depression, cognitive dysfunction and dementia [Citation3–5]. Many previous studies have shown that hypertension is one of the most important factors for the presence of WMLs [Citation8–10]. Hypertension could cause damage of the media and thickening of the vessel walls, which result in narrowing of the small perforating arteries and arterioles in white matter [Citation25]. The anastomotic system of perforating artery is very poor, so white matter is vulnerable to cerebral ischemia, which is the main pathogenesis of WMLs [Citation25]. Elevated BP may also damage endothelial blood-brain barrier directly, which results in cerebral parenchymal damage [Citation26].
WMLs can be detected even in normotensive subjects. In the ARIC study reporting 24.6% prevalence of WMLs, 51% of subjects with WMLs were normotensive [Citation1]. In the Rotterdam Study, the prevalence was 27% and 61% of whom were also normotensive [Citation27]. Resting BP has been suggested as the baseline for treatment of hypertension, but it may not be sufficient for assessing BP in the daily life [Citation28]. Other markers, derived from ambulatory BP monitoring or treadmill test, could be more related with target organ damages and cardiovascular risk [Citation15,Citation29]. As to small vessel disease of brain, resting office BP also may not be sufficient for risk stratification. In this study, we firstly demonstrated the significant relationship of peak exercise BP to WMLs, independent of resting BP in normotensive subjects.
Blood pressure during treadmill test could reflect hemodynamics during daily physical activity and provide additive information not available from resting BP. An exaggerated BP response to exercise could lead to target organ damages such as LV hypertrophy and carotid atherosclerosis [Citation20,Citation21]. Recently we demonstrated that exaggerated BP response was associated with subclinical myocardial dysfunction even in normotensive individuals [Citation22]. Intermittent BP elevation due to physical activity or mental stress could have adverse effects on target organs including the brain. As like resting BP, intermittent BP elevation during physical activity and exercise could influence on small vessels of brain in the same way. One previous study reported that systolic BP rise during exercise as well as baseline BP was related with the risk of stroke [Citation16]. Recent other study with long-term follow-up of the healthy men have reported the association of elevated systolic BP during exercise with stroke [Citation19]. As to subclinical small vessel disease of brain, we found similar results and demonstrated the importance of BP response during exercise. In this study, even normotensive subjects had higher risk for cerebral small vessel disease in the presence of elevated BP response during exercise. Unlike previous studies, brain MRI and treadmill tests were performed consecutively in asymptomatic participants. The results of this study suggest that elevated BP during exercise could be a marker of subclinical atherosclerotic diseases.
The mechanism of exaggerated BP response and linkage with WMLs is not clear yet. Vascular function, activities of the sympathetic nervous system and rennin-angiotensin-aldosterone system have been suggested as possible mechanisms for exaggerated BP response to exercise [Citation15]. During exercise, cardiac output increases to support working muscle by sympathetic activity. The BP response to the increased cardiac output is regulated by peripheral resistance via endothelium-dependent vasodilation [Citation30]. Endothelial dysfunction, failure of endothelium dependent vasodilation, would reduce the normal fall in peripheral vascular resistance leading to significant systolic BP elevation during exercise [Citation15]. A high intraluminal pressure could subsequently induce endothelial damage of the blood-brain barrier and lead to cerebral WMLs. In addition, endothelial dysfunction in the peripheral extremities and brain might develop concurrently as a systemic reaction.
The exercise test is usually performed when ischemic heart disease or arrhythmia is suspected. Although many previous studies have reported the association of exercise BP with cardiovascular risk, the exercise test is not recommended as a routine screening test. The evidence for treatment of elevated BP during exercise is also insufficient. Nonetheless, hemodynamic variables during exercise test could be useful to identify the risk for current subclinical atherosclerotic disease and future cardiovascular disease.
Study limitations
This study had several limitations. First, there could be selection bias. This study was conducted with selected individuals who visited for health check-up. In many cases, the degree of WMLs was mild. So, we should be cautious in generalization of our findings. Second, we used 1.5-T MRI, which has shown less sensitivity in detecting WMLs compared to 3-T MRI. 3-T MRI revealed a greater number and volume of WMLs in a previous study. We did not analyze the volume of WMLs and association between their degree and exercise BP, as the degree was mild in many cases. We only analyzed the association between exercise BP and the presence of WMLs. With 3-T MRI, further analysis according to the degree of WMLs might be possible, and would provide more objective information. Third, we did not evaluate 24 hour ambulatory BP monitoring. So, we cannot completely rule out the possibility that patients with masked hypertension were enrolled. Finally, the cross-sectional study design and relatively small number of subjects are also potential limitations. However, brain MRI and treadmill tests were performed consecutively and the results of this study suggest that elevated BP during exercise could be a marker of current subclinical atherosclerotic diseases.
In conclusion, baseline BP is a very important risk factor for WMLs, subclinical small vessel disease of brain. However, even normotensive subjects have a risk of WMLs and peak systolic BP during exercise is closely related to WMLs in these normotensive subjects. Exercise test may provide additional information regarding risk factors for target organ damage including brain. Based on this study, close observation is recommended even for the normotensive subjects with an elevated BP during exercise, especially those with additional cardiovascular risk factors.
Disclosure statement
We have no conflicts of interest to declare.
References
- Liao D, Cooper L, Cai J, et al. Presence and severity of cerebral white matter lesions and hypertension, its treatment, and its control. The ARIC Study. Atherosclerosis Risk in Communities Study. Stroke 1996;27:2262–2270.
- de Leeuw FE, de Groot JC, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry. 2001;70:9–14.
- Vermeer SE, Hollander M, van Dijk EJ, et al. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003;34:1126–1129.
- Vermeer SE, Prins ND, den Heijer T, et al. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222.
- Salloway S, Malloy P, Kohn R, et al. MRI and neuropsychological differences in early- and late-life-onset geriatric depression. Neurology. 1996;46:1567–1574.
- Braffman BH, Zimmerman RA, Trojanowski JQ, et al. Brain MR: pathologic correlation with gross and histopathology. 2. Hyperintense white-matter foci in the elderly. AJR Am J Roentgenol. 1988;151:559–566.
- van Swieten JC, van den Hout JH, van Ketel BA, et al. Periventricular lesions in the white matter on magnetic resonance imaging in the elderly. A morphometric correlation with arteriolosclerosis and dilated perivascular spaces. Brain. 1991;114:761–774.
- Swan GE, DeCarli C, Miller BL, et al. Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology. 1998;51:986–993.
- Dufouil C, de Kersaint-Gilly A, BesanÃon V, et al. Longitudinal study of blood pressure and white matter hyperintensities: the EVA MRI Cohort. Neurology. 2001;56:921–926.
- van Dijk EJ, Breteler MM, Schmidt R, et al. The association between blood pressure, hypertension, and cerebral white matter lesions: cardiovascular determinants of dementia study. Hypertension. 2004;44:625–630.
- Godin O, Tzourio C, Maillard P, et al. Antihypertensive treatment and change in blood pressure are associated with the progression of white matter lesion volumes: the Three-City (3C)-Dijon Magnetic Resonance Imaging Study. Circulation. 2011;123:266–273.
- Verhaaren BF, Vernooij MW, de Boer R, et al. High blood pressure and cerebral white matter lesion progression in the general population. Hypertension. 2013;61:1354–1359.
- Gouw A, van der Flier WM, Fazekas F, et al. Progression of white matter hyperintensities and incidence of new lacunes over a 3-year period: the Leukoaraiosis and Disability study. Stroke. 2008;39:1414–1420.
- Weber R, Weimar C, Blatchford J, et al. Telmisartan on top of antihypertensive treatment does not prevent progression of cerebral white matter lesions in the prevention regimen for effectively avoiding second strokes (PRoFESS) MRI substudy. Stroke. 2012;43:2336–2342.
- Thanassoulis G, Lyass A, Benjamin EJ, et al. Relations of exercise blood pressure response to cardiovascular risk factors and vascular function in the Framingham Heart Study. Circulation. 2012;125:2836–2843.
- Kurl S, Laukkanen JA, Rauramaa R, et al. Systolic blood pressure response to exercise stress test and risk of stroke. Stroke. 2001;32:2036–2041.
- Weiss SA, Blumenthal RS, Sharrett AR, et al. Exercise blood pressure and future cardiovascular death in asymptomatic individuals. Circulation. 2010;121:2109–2116.
- Mariampillai JE, Engeseth K, Kjeldsen SE, et al. Exercise systolic blood pressure at moderate workload predicts cardiovascular disease and mortality through 35 years of follow-up in healthy, middle-aged men. Blood Press. 2017;26:229–236.
- Prestgaard E, Hodnesdal C, Engeseth K, et al. Long-term predictors of stroke in healthy middle-aged men. Int J Stroke. 2017. doi: 10.1177/1747493017730760. [Epub ahead of print]
- Gottdiener JS, Brown J, Zoltick J, et al. Left ventricular hypertrophy in men with normal blood pressure: relation to exaggerated blood pressure response to exercise. Ann Intern Med. 1990;112:161–166.
- Jae SY, Fernhall B, Heffernan KS, et al. Exaggerated blood pressure response to exercise is associated with carotid atherosclerosis in apparently healthy men. J Hypertens. 2006;24:881–887.
- Yang W, Shim CY, Bang WD, et al. Asynchronous arterial systolic expansion as a marker of vascular aging: assessment of the carotid artery with velocity vector imaging. J Hypertens. 2011;29:2404–2412.
- Fazekas F, Chawluk JB, Alavi A, et al. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–356.
- Kivipelto M, Soininen H, Tuomilehto J. Hypertension and white matter lesions of the brain. J Hypertens. 2002;20:387–389.
- Pantoni L, Garcia JH. The significance of cerebral white matter abnormalities 100 years after Binswanger’s report. A review. Stroke. 1995;26:1293–1301.
- Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 2013;12:483–497.
- Breteler MM, van Swieten JC, Bots ML, et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: the Rotterdam Study. Neurology. 1994;44:1246–1252.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–2219.
- Clement DL, De Buyzere ML, De Bacquer DA, et al. Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N Engl J Med. 2003;348:2407–2415.
- Le V, Mitiku T, Sungar G, et al. The blood pressure response to dynamic exercise testing: a systematic review. Progr Cardiovasc Dis. 2008;51:135–160.