Abstract
Purpose: Only few studies evaluated biomarkers useful for defining the cardiovascular risk of a subject in a pre-clinical condition (i.e. healthy subjects). In this context we sought to determine the relationships of Plasminogen activator inhibitor type 1 (PAI‐1), P-Selectin, Tissue Inhibitors Metalloproteinases type 1 (TIMP-1) and Cystatin-C with subclinical Target Organ Damage (TOD) in normotensive and normoglycemic subjects without known cardiovascular and kidney diseases.
Materials and Methods: 480 blood donors participated at the present analysis. TOD was evaluated as Pulse Wave Velocity (PWV), Left Ventricular Hypertrophy (LVH) and Intima Media Thickness (IMT) and carotid plaque presence) grouped together under carotid TOD.
Results: 3.1% of the subjects showed a PWV higher than 10 m/sec with those subjects exerting significantly lower values of P-Selectine (0.068 ± 0.015 vs 0.08 ± 0.036 mg/L, p = .014). 8.8% of the subjects showed carotid TOD that was associated with higher Cystatin-C values (0.67 ± 0.17 vs 0.63 ± 0.14 mg/L, p = .045). Finally 23.8% of the subjects showed LVH with no significant differences regarding biomarkers. Despite some significant correlations between biomarkers and TOD, at the multivariate analysis none came out to be as significant predictor of the assessed TOD.
Conclusions: in normotensive and normoglycemic healthy subjects, the evaluated biomarkers of atherosclerotic process didn’t show any significant association with cardiac, carotid and vascular TOD while age and BP are its principal predictors.
Introduction
Research on biomarkers of help in defining cardiovascular (CV) risk of a subject has increased dramatically in recent years [Citation1,Citation2]. To be defined useful a biomarkers must meet three criteria: (1) accuracy, (2) reliability and (3) diagnostic or therapeutic impact [Citation3]. While the first two characteristics has been shown for many biomarkers, only few of them demostrated a clinical impact and are effectively used (i.e proBNP or Troponin). Furthermore those biomarkers has been widely evaluated in patients with hypertension [Citation4–6], chronic kidney disease [Citation7], previous myocardial infarction [Citation8] or stroke [Citation9], while only few of them focus on healthy subjects [Citation10–13] that are those who would benefit most from the early definition of a high cardiovascular risk and so early and strong therapeutical management. Since studies based on hard end-points are very long, expensive and require a large number of patients, many studies used surrogate end-point, such as target organ damage (TOD), in research on this topic [Citation4–7,Citation10–13].
In this context we sought to determine the role of some biomarkers already identified to present an important role in the atherosclerotic process in normotensive and normoglycemic subjects without known CV and kidney diseases. The biomarkersinclude: (1) Plasminogen activator inhibitor type 1 (PAI‐1) [Citation14]; (2) P-Selectin, [Citation15]; (3) Tissue Inhibitors Metalloproteinases type 1 (TIMP-1) [Citation16] and finally (4) Cystatin-C [Citation17,Citation18]. We analyzed their correlation with subclinical TOD evaluated as vascular (i.e. Intima Media Thickness - IMT -, carotid plaque and Pulse Wave Velocity – PWV) and cardiac one (Left Ventricular Hypertrophy – LVH).
Material and methods
Study population
From September 2009 to January 2012, we enrolled 756 consecutive 18–80 aged taken from the blood donor list of the S. Gerardo Hospital (Monza, Italy) and the Desio Hospital (Desio, Italy) without known CV and kidney diseases. In 480 of these subjects the four reported biomarkers were evaluated.
Main exclusion criteria were age less than 18 years old, pregnancy, hypertension (defined as the evidence of a Blood Pressure – BP higher than 140/90 mmHg on the enrolment visit or as the reported use of antihypertensive drugs), diabetes mellitus (defined as the evidence of a glucose higher than 126 mg/dL or as the reported use of antidiabetic drugs), chronic kidney diseases (defined as a eGFR less than 60 mL/min or the presence of microalbuminuria) and any history of CV events (myocardial infarction, angina pectoris, heart failure, stroke, transient ischemic attacks and claudication). Furthermore also chronic pulmonary disease, substance abuse and history of cancer were exclusion criteria.
In all subjects, we collect a comprehensive medical history and performed a complete physical examination. With the patient in the sitting position for at least 5 min and with the arm placed at heart level, two semi-automated BP measurements were taken (OMRON Helthcare Europe, Hoofddorp, The Netherlands) and the average was used for statistics.
Measurements included fasting serum glucose, serum total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), cholesterol, serum triglycerides and creatinine. A urine dip-stick was done in order to exclude micro-albuminuria. Glomerular Filtration Rate was estimated (eGFR) by the Modification of Diet in Renal Disease (MDRD) equation [Citation19]. Height and weight were obtained to calculate Body Mass Index (BMI) and waist circumference was assessed halfway between the lower ribs and the iliac crest.
Biomarkers measurement
Blood samples were withdrawn from the left antecubital vein and stored at −20 °C. Blood was centrifugated and plasma aspirated and stored at −80 °C. In 480 patients PAI‐1, P-Selectin, TIMP-1 and Cystatin-C were measured and used in the present analysis. No significant differences were found between patients of the present analysis and the whole population.
Biomarkers have been assessed using Performance Assay Luminex, with polystyrene beads, and BIORAD BIO-PLEX 100. Values of the standard curves were compared with the values provided by the manufacturer, and must not exceed a CV of 15%. The sensitivity were 9.96 × 10−6 mg/l, 6.74 × 10−5 mg/L, 1.75 × 10−5 mg/l and 4.69 × 10−4 mg/L for PAI-1, P-Selectin, TIMP-1 and Cystatin-C, respectively.
Pulse wave velocity
Aortic stiffness was evaluated by PWV between the carotid and the femoral artery of the same side with the patient in the supine position. The pressure pulse waveforms were simultaneously obtained at the two arterial sites at the right side using an automatic device (Complior, Colson; Alam Medical, Paris, France) and their distance calculated by taking the distance between hip and neck via a rigid ruler. Measurement was corrected by a 0.8 factor accordingly to the PWV measurement methods consensus documents which states to use the subtraction methods instead of the direct one when assessing the distance between the two measurements points [Citation20]. Two measurements were obtained in each patient and the mean was used for the analysis. In our laboratory the intra-session within- and between-operator variability of PWV amounts respectively to a coefficient of variation of the mean value of 2% and to 4%, the corresponding value for the inter-session between-operator variability being 4%.
Arterial stiffness was defined as a PWV measurement higher than 10 m/sec accordingly to current guidelines [Citation20].
Left ventricular mass
As previously published [Citation21], two-dimensional echocardiograms were performed by an experienced cardiologist using a dedicated ultrasound machine (SONOS 5500; Philips Healthcare, Andover, Massachusetts, USA with an ultrasound transducer of 2.5MHz) in each patient. 2D high frame rate gray-scale loops of four-chamber, two-chamber, and three-chamber views with average frame rate of 90 frames per second (fps) were used in order to measure left ventricular end-diastolic diameter (LVEDD), interventricular septum and posterior wall thickness. Left Ventricular Mass (LVM) was calculated using Devereux formulae [Citation22]. The LVM values were normalized for Body Surface Area (BSA) to obtain Left Ventricular Mass Index (LVMI). BSA was calculated by using the DuBois and DuBois formulae: BSA (m2) = 0.007184 × height (cm)0.725 × weigh (kg)0.425. The intra-operator variability in terms of coefficient of variation of the mean of two measurements is less than 3% in our laboratory. LVH was diagnosed by LVMI of at least 115 g/m2 for men and at least 95 g/m2 in women [Citation23].
Intima media thickness and carotid plaque
With the subject in the supine position and the neck in partial extension, we scanned right carotid artery through an ultrasonography device (Philips Sonos 5500). The transducer was manually oriented perpendicularly to the longitudinal axis of the vessel under B-mode guidance and common carotid IMT was measured at a posterior wall site located 2 cm below bifurcation as the difference between the inner ipoechogenic and the middle anechogenic layers [Citation24]. Measurements were made by two operators unaware of the subject’s clinical status, two measurements were obtained in each patient and the mean was used for the analysis. In our laboratory the intra-session within- and between-operator variability of IMT amounts respectively to a coefficient of variation of the mean value of 2.5% and to 2%, the corresponding value for the inter-session between-operator variability being 3.9%. Carotid plaque was defined as the presence of a IMT >1.2 mm in the common carotid, bulb or internal carotid artery. Finally Carotid TOD was defined as the presence of an IMT >0.9 mm or the presence of a carotid plaque.
Statistical analysis
Data obtained in each subject were averaged, and individual data were summed and expressed as means (±SD). Between-group differences were assessed by Student t, Mann-Whitney test and χ2 tests (or Fisher exact test when needed) for normally distributed, non-normally distributed and categorical variables, respectively. Age-adjusted analysis were performed as needed. Pearson’s or Spearman’s correlation coefficients were used, as appropriate, to test the association between variables. We performed linear regression using the additive model and adjusting for covariates determined by stepwise regression. We used PWV, LVMI and IMT as the dependent variables with PAI‐1, P-Selectin, TIMP-1,Cystatin-C, age, sex, SBP, waist circumference, total cholesterol, glucose and eGFR as covariates for multivariate adjustments.
SPSS 13.0 (SPSS, IBM, United States) was used for the statistical analyses and a p value < .05 was taken as the level of statistical significance.
Ethical considerations
The study protocol complies with the Declaration of Helsinki and was approved by the Ethics Committee of the Institutions involved. All participants provided informed written consent after being informed of the study nature and purpose.
Results
Population characteristics
Table S1 reports the main demographic, clinical, biochemical and TOD characteristics of the whole population. Mean age was 44.9 ± 9.6 years and, accordingly to our inclusione criteria no patients presents hypertension (mean Systolic and Diastolic BP were 120.8 ± 11.8 and 76.0 ± 8.6 mmHg, respectively) or diabetes mellitus (mean glucose 89.9 ± 11.1 mg/dL). LDL cholesterol was 122.4 ± 30.6 mg/dL while triglycerides were 93.0 ± 47.5 mg/dL. Finally no chronic kidney disease was dected with a mean creatinine 0.88 ± 0.18 mg/dL (with an eGFR of 96.0 ± 17.4 mL/min) and with no patients showing microalbuminuria on urine dip-stick.
Table S1 also reports the values of PAI‐1, P-Selectin, TIMP-1 and Cystatin-C. Finally, regarding TOD, mean PWV was 7.6 ± 1.2 m/sec, carotid IMT 0.62 ± 0.13 mm and LVMI 96.8 ± 22.6 g/m2. Accordingly, a PWV higher than 10 m/sec was more found in 15 subjects (3.1%), LVH was showed by 23.8% (114 subjects) while carotid TOD was found in 42 patients (8.8%). In fact, while a substantial proportion of subjects showed the presence of at least one TOD (31.5%), only in 2.1% two or more TOD are present at the same time.
Biomarkers of atherosclerotic process and carotid TOD
showed demographic, clinical and biochemical characteristics when patients were divided accordingly to the presence or absence of carotid TOD. Subjects with carotid involvement were older (52.2 ± 7.5 vs 44.2 ± 9.5 years, p < .001), with higher glucose and total cholesterol levels (94.2 ± 13.0 vs 89.5 ± 10.8 mg/dL and 208.1 ± 28.9 vs 194.4 ± 33.0 mg/dL, respectively; p < .05 for both comparison), while no differences were seen for BP values, BMI, fractioned cholesterol and tryglicerides and renal function. Regarding biomarkers only Cystatin-C showed a significant difference with higher values in the group with TOD (0.67 ± 0.17 vs 0.63 ± 0.14 mg/L) that was confirmed in the age-adjusted analysis (p = .045; ).
Figure 1. Values of the 4 markers of atherosclerotic process depending on carotid target organe damage (panel A), arterial stiffness (panel B) and left ventricular hypertrophy (panel C). PWV: Pulse Wave Velocity; PAI: Plasminogen Activator Inhibitor; TIMP: Tissue Inhibitor Metalloproteinases. Vertical lines showed 5th and 95th percentiles, boxes shown 25th and 75th percentiles, horizontal lines shown 50th percentile.
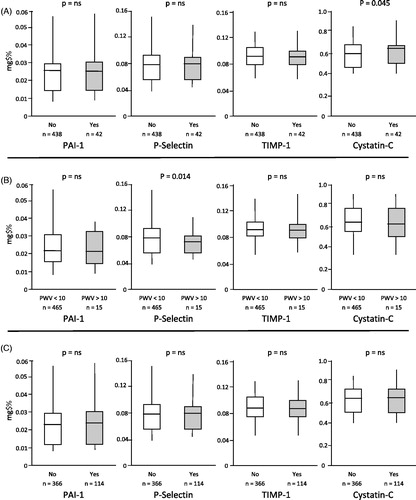
Table 1. Demographic, clinical and biochemical characteristics when patients were divided accordingly to the presence of carotid target organ damage.
Biomarkers of atherosclerotic process and arterial stiffness
showed demographic, clinical and biochemical characteristics when patients were divided accordingly to the presence or absence of arterial stiffness. Subjects with PWV >10 m/s presents higher Systolic and Diastolic BP (129.9 ± 17.9 vs 120.5 ± 11.4 and 80.9 ± 9.8 vs 75.9 ± 8.5, respectively; p < .05 for both comparison) while age, lipids, glucose BMI and renal function were superimposable between the two groups. Regarding biomarkers, only P-Selectin showed a significant difference with lower values in the group with TOD (0.068 ± 0.015 vs 0.08 ± 0.036 mg/L, p = .014; ).
Table 2. Demographic, clinical and biochemical characteristics when patients were divided accordingly to the presence of arterial stiffness.
Biomarkers of atherosclerotic process and LVH
shows demographic, clinical and biochemical characteristics when patients were divided accordingly to the presence or absence of cardiac TOD. Subjects with LVH were older (48.2 ± 9.4 vs 43.9 ± 9.5 years, p < .001), with higher systolic and diastolic BP (124.5 ± 13.1 vs 119.6 ± 11.1 and 77.8 ± 8.0 vs 75.5 ± 8.7, respectively; p < .05 for both comparison) while lipids, glucose and renal function were superimposable. Also regarding biomarkers no difference was detected between the two groups ().
Table 3. Demographic, clinical and biochemical characteristics when patients were divided accordingly to the presence of left ventricular hypertrophy.
Correlation, regression and multivariate analysis
As shown in Table S2 Cystatin-C correlated with LVMI (r = 0.1, p = .02) and IMT (r= 0.14, p < .01), while TIMP-1 correlated only with IMT (r = 0.11, p = .02). P-Selectin and PAI-1 didn’t show any significant correlation. At the multivariate analysis none of the evaluated biomarkers resulted to be among the significant predictors of the assessed TOD. Anyway, with a total r2 of 0.13, age (β = 0.16, p = .001), sex (β = 0.12, p = .014) and Systolic BP (β = 0.25, p < .001) were the independent predictors of PWV at the multivariate analysis. For IMT the only significant independent predictor was age (β = 0.33, p < .001, total r2 = 0.17). Finally, LVMI showed at the multivariate analysis age (β = 0.18, p < .001), sex (β = 0.40, p < .001) and systolic BP (β = 0.136, p = .003) as the independent predictors with a total r2 of 0.24.
Discussion
In our normotensive and normoglycemic population without known cardiovascular and kidney diseases, the evaluated biomarkers of atherosclerotic process didn’t show significant association with cardiac, carotid and vascular TOD. In fact, despite some small differences when subjects were divided accordingly to the presence/absence of TOD and some correlation were found, at the multivariate analysis none of the evaluated biomarkers results to be among the significant predictors of the assessed TOD.
The biomarkers we chosen to evaluate are all implicated into the atherosclerotic process at various levels. Inflammation is a key event at the beginning of atherosclerosis [Citation25] with proinflammatory citokines and cell adhesion molecules (such as P-Selectin) that, through reduced nitric oxide bioavailability and increased production of reactive oxygen species are able to determine various pathways activation. Among them, the MMP/TIMP system plays a key role in matrix degradation, while Cystatin-C acts on cathepsins S and K, molecules implicated in inflammatory process and overexpressed in atherosclerotic lesions. Finally, PAI-1 is fundamental in the process of atherothrombosys in which it exert an important regulatory role [Citation14]. In fact, all of these molecules have been related to CV fatal and non-fatal events [Citation8,Citation26–28] but the underlying mechanism, above the molecular one, has not yet been entirely clarified. One possible mechanism could be that the biochemical pathways above outlined act determining vascular and cardiac TOD. At the vascular level, all these pathways are able to determine a progressive decrease in elastin/collagen ratio, reduced smooth muscle cell proliferation and impaired endothelial mediated vasodilation that finally leads to arterial stiffness and atherosclerosis. At cardiac level similar mechanisms are implicated in LVH characterized by a complex remodelling of the myocardial structure such as enhanced cardiomyocyte growth, excessive cardiomyocyte apoptosis and accumulation of interstitial and perivascular collagen fibres [Citation29].
Results regarding the evaluated biomarkers and TOD are very heterogeneous. The majority of studies are concentrated on carotid TOD, where a preclinical evidence of a higher concentration of PAI-1 into carotid plaque and its relationship with its instability [Citation30] has been confirmed by the finding of association with IMT measurements in hypertensive patients [Citation4]. Contrariwise, P-Selectin doesn’t correlate with plaque inflammation [Citation31], while it has been associated with IMT increase in some but not all studies [Citation32,Citation33]. Same figures of heterogeneity were showed also for TIMP-1 [Citation7,Citation13,Citation34] and Cystatin-C [Citation35,Citation36]. Taking into account only studies on general population, only PAI-1 has been found to correlate with IMT [Citation10] while the other markers do not [Citation12,Citation37]. Regarding arterial stiffness, conflicting results have been found for PAI-1 [Citation38], and TIMP-1 [Citation6,Citation34,Citation39]. P-Selectin didn’t show any correlation with arterial stiffness [Citation40] while Cystatine-C seems to correlate, with various degree, in hypertensive patients and subjects with chronic kidney diseases [Citation41–44]. Experiences on general population are limited to PAI-1 in which no association with PWV was seen [Citation11]. Finally, no association was seen betyween LVH, PAI-1 [Citation45] and TIMP-1 [Citation46] while it was for P-Selectin in hypertensive [Citation47] and elderly subjects [Citation48] and for Cystatine-C in the general population [Citation49].
The difference in results could be easily explained by the important heterogeneity in populations examined, which included hypertensive patients [Citation6,Citation35,Citation41] dislipidemic [Citation32], obese [Citation33], patients with previous stroke [Citation38], gestational diabetes [Citation39] and metabolic syndrome [Citation36]. Furthermore, TOD are evaluated in different ways among studies: for example regarding arterial stiffness some assess carotid-femoral PWV [Citation41,Citation44] while other used brachial-ankle PWV [Citation42] or Cardio-Ankle Vascular-Index [Citation43].
Our study has some limitations. First it has a cross-sectional design, which means that it does not provide evidence on progression of the TOD and its association with evaluated biomarkers. Second, we did not obtain a comprehensive assessment of atherosclerosis biomarkers and thus could not provide a definitive description of the relationship of these markers with cardiac and vascular TOD. Furthermore, since patients were relatively healthy some subgroups of TOD were small thus limiting statistical analysis. Finally, microalbuminuria was excluded only by urine dip-stick, while only a 24 h dosage could definitively exclude the presence of very little protein urinary excretion.
Our study, however, has also elements of strength.these include the fact that TOD has been measured by state of the art techniques. Furthermore, our subjects are blood donors normotensive and normoglycemic and free from any cardiovascular, metabolic and kidney overt disease.
In conclusion, in our normotensive and normoglycemic population without known cardiovascular and kidney diseases, the evaluated biomarkers of atherosclerotic process (PAI-1, P-Selectin, TIMP-1 and Cystatin-C) didn’t show any significant association with cardiac, carotid and vascular TOD. In these subjects, the greater predictor of target organ damage are the classical risk factors, ad in particular age and systolic blood pressure.
Table_S1-S2.docx
Download MS Word (17.7 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Shemisa K, Bhatt A, Cheeran D, et al. Novel biomarkers of subclinical cardiac dysfunction in the general population. Curr Heart Fail Rep. 2017;14:301–310.
- Wang J, Tan GJ, Han LN, et al. Novel biomarkers for cardiovascular risk prediction. J Geriatr Cardiol. 2017;14:135–150.
- Redberg RF, Vogel RA, Criqui MH, et al. 34th Bethesda conference: task force #3––what is the spectrum of current and emerging techniques for the noninvasive measurement of atherosclerosis? J Am Coll Cardiol. 2003;41:1886–1898.
- Marchesi E, Martignoni A, Tinelli C, et al. Plasminogen activator inhibitor-1 and carotid intima-media thickening in patients with newly detected primary hypertension. J Cardiovasc Risk. 1999;6:363–369.
- Kondo K, Kitagawa K, Nagai Y, et al. Associations of soluble intercellular adhesion molecule-1 with carotid atherosclerosis progression. Atherosclerosis. 2005;179:155–160.
- Tan J, Hua Q, Xing X, et al. Impact of the metalloproteinase-9/tissue inhibitor of metalloproteinase-1 system on large arterial stiffness in patients with essential hypertension. Hypertens Res. 2007;30:959–963.
- Kousios A, Kouis P, Panayiotou AG. Matrix metalloproteinases and subclinical stherosclerosis in chronic kidney disease: a systematic review. Int J Nephrol. 2016;2016:9498013.
- Hamsten A, de Faire U, Walldius G, et al. Plasminogen activator inhibitor in plasma: risk factor for recurrent myocardial infarction. Lancet. 1987;2:3–9.
- The National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587.
- Sakata T, Mannami T, Baba S, et al. Potential of free-form TFPI and PAI-1 to be useful markers of early atherosclerosis in a Japanese general population (the Suita Study): association with the intimal-medial thickness of carotid arteries. Atherosclerosis. 2004;176:355–360.
- Nishiwaki Y, Takebayashi T, Omae K, et al. Relationship between the blood coagulation-fibrinolysis system and the subclinical indicators of arteriosclerosis in a healthy male population. J Epidemiol. 2000;10:34–41.
- Thakore AH, Guo CY, Larson MG, et al. Association of multiple inflammatory markers with carotid intimal medial thickness and stenosis (from the Framingham Heart Study). Am J Cardiol. 2007;99:1598–1602.
- Romero JR, Vasan RS, Beiser AS, et al. Association of carotid artery atherosclerosis with circulating biomarkers of extracellular matrix remodeling: the Framingham Offspring Study. J Stroke Cerebrovasc Dis. 2008;17:412–417.
- Song C, Burgess S, Eicher JD, et al. Causal effect of plasminogen activator inhibitor type 1 on coronary heart disease. J Am Heart Assoc. 2017;6:e004918.
- Dong ZM, Chapman SM, Brown AA, et al. The combined role of P- and E-selectins in atherosclerosis. J Clin Invest. 1998;102:145–152.
- Tan RJ, Liu Y. Matrix metalloproteinases in kidney homeostasis and diseases. Am J Physiol Renal Physiol. 2012;302:F1351–F1361.
- Shlipak MG, Praught ML, Sarnak MJ. Update on cystatin C: new insights into the importance of mild kidney dysfunction. Curr Opin Nephrol Hypertens. 2006;15:270–275.
- Salgado JV, Souza FL, Salgado BJ. How to understand the association between cystatin C levels and cardiovascular disease: imbalance, counterbalance, or consequence? J Cardiol. 2013;62:331–335.
- Modification of Diet in Renal D. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–772.
- The Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J. 2010;31:2338–2350.
- Maloberti A, Meani P, Vallerio P, et al. Annexin A5 in treated hypertensive patients and its association with target organ damage. J Hypertens. 2017;35:154–161.
- Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–618.
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270.
- Maloberti A, Meani P, Varrenti M, et al. Structural and Functional Abnormalities of Carotid Artery and Their Relation with EVA Phenomenon. High Blood Press Cardiovasc Prev. 2015;22:373–379.
- Park S, Lakatta EG. Role of inflammation in the pathogenesis of arterial stiffness. Yonsei Med J. 2012;53:258–261.
- Ridker PM, Buring JE, Rifai N. Soluble P-selectin and the risk of future cardiovascular events. Circulation. 2001;103:491–495.
- Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–2060.
- Kormi I, Nieminen MT, Havulinna AS, et al. Matrix metalloproteinase-8 and tissue inhibitor of matrix metalloproteinase-1 predict incident cardiovascular disease events and all-cause mortality in a population-based cohort. Eur J Prev Cardiolog. 2017;24:1136–1144.
- Dıez J, Gonzalez A, Lopez B, et al. Mechanisms of disease: pathologic structural remodelling is more than adaptive hypertrophy in hypertensive heart disease. Nat Clin Pract Cardiovasc Med. 2005;2:209–216.
- Ekmekçi H, Güngör Öztürk Z, Ekmekçi OB, et al. Significance of vitronectin and PAI-1 activity levels in carotid artery disease: comparison of symptomatic and asymptomatic patients. Minerva Med. 2013;104:215–223.
- Duivenvoorden R, Mani V, Woodward M, et al. Relationship of serum inflammatory biomarkers with plaque inflammation assessed by FDG PET/CT: the dal-PLAQUE study. JACC Cardiovasc Imaging. 2013;6:1087–1094.
- Guardamagna O, Abello F, Saracco P, et al. Endothelial activation, inflammation and premature atherosclerosis in children with familial dyslipidemia. Atherosclerosis. 2009;207:471–475.
- Csongrádi É, Káplár M, Nagy B, et al. Adipokines as atherothrombotic risk factors in obese subjects: Associations with haemostatic markers and common carotid wall thickness. Nutr Metab Cardiovasc Dis. 2017;27:571–580.
- Zureik M, Beaudeux JL, Courbon D, et al. Serum tissue inhibitors of metalloproteinases 1 (TIMP-1) and carotid atherosclerosis and aortic arterial stiffness. J Hypertens. 2005;23:2263–2268.
- Watanabe S, Okura T, Liu J, et al. Serum cystatin C level is a marker of end-organ damage in patients with essential hypertension. Hypertens Res. 2003;26:895–899.
- Huang R, Gu J, Cao Q, et al. The association between serum cystatin C and carotid intima-media thickness in metabolic syndrome patients with normal estimated glomerular filtration rate. Clin Chim Acta. 2015;448:170–173.
- Yamashita H, Nishino T, Obata Y, et al. Association between cystatin C and arteriosclerosis in the absence of chronic kidney disease. J Atheroscler Thromb. 2013;20:548–556.
- Tuttolomondo A, Di Raimondo D, Pecoraro R, et al. Immune-inflammatory markers and arterial stiffness indexes in subjects with acute ischemic stroke. Atherosclerosis. 2010;213:311–318.
- Vilmi-Kerälä T, Lauhio A, Tervahartiala T, et al. Subclinical inflammation associated with prolonged TIMP-1 upregulation and arterial stiffness after gestational diabetes mellitus: a hospital-based cohort study. Cardiovasc Diabetol. 2017;16:49.
- Yamasaki F, Furuno T, Sato K, et al. Association between arterial stiffness and platelet activation. J Hum Hypertens. 2005;19:527–533.
- Ozkok A, Akpinar TS, Tufan F, et al. Cystatin C is better than albuminuria as a predictor of pulse wave velocity in hypertensive patients. Clin Exp Hypertens. 2014;36:222–226.
- Song SH, Kwak IS, Kim YJ, et al. Serum cystatin C is related to pulse wave velocity even in subjects with normal serum creatinine. Hypertens Res. 2008;31:1895–1902.
- Nakamura K, Iizuka T, Takahashi M, et al. Association between cardio-ankle vascular index and serum cystatin C levels in patients with cardiovascular risk factor. J Atheroscler Thromb. 2009;16:371–379.
- Odaira M, Tomiyama H, Matsumoto C, et al. Association of serum cystatin C with pulse wave velocity, but not pressure wave reflection, in subjects with normal renal function or mild chronic kidney disease. Am J Hypertens. 2010;23:967–973.
- Gidding SS, Palermo RA, DeLoach SS, et al. Associations of cardiac structure with obesity, blood pressure, inflammation, and insulin resistance in African-American adolescents. Pediatr Cardiol. 2014;35:307–314.
- Ishikawa J, Kario K, Matsui Y, et al. Collagen metabolism in extracellular matrix may be involved in arterial stiffness in older hypertensive patients with left ventricular hypertrophy. Hypertens Res. 2005;28:995–1001.
- de Faria AP, Ritter AM, Sabbatini AR, et al. Deregulation of soluble adhesion molecules in resistant hypertension and its role in cardiovascular remodeling. Circ J. 2016;80:1196–1201.
- Masiha S, Sundström J, Lind L. Inflammatory markers are associated with left ventricular hypertrophy and diastolic dysfunction in a population-based sample of elderly men and women. J Hum Hypertens. 2013;27:13–17.
- Moran A, Katz R, Jenny NS, et al. Left ventricular hypertrophy in mild and moderate reduction in kidney function determined using cardiac magnetic resonance imaging and cystatin C: the multi-ethnic study of atherosclerosis (MESA). Am J Kidney Dis. 2008;52:839–848.
