Abstract
Background: Arterial hypertension is associated with obstructive sleep apnoea, poor quality and duration of sleep, which might contribute to hypertension-mediated organ damage.
Methods: We investigated the presence of insomnia, restless legs syndrome, and obstructive sleep apnoea using validated questionnaires (Insomnia Severity Index, Restless Legs Syndrome Rating Scale, and STOP-Bang), and their relationship with hypertension-mediated organ damage, in hypertensive patients.
Results: In 159 consecutive consenting hypertensive patients [age 47(11) years, median and (interquartile range), body mass index 25.5(5.9) kg/m2, office systolic and diastolic blood pressure 144(23)/92(12) mmHg], the STOP-Bang, but not the other scores, predicted cardiac remodelling: compared to patients with a STOP-Bang score < 3, those at high risk of obstructive sleep apnoea showed higher left ventricular mass index [49.8(11.9) vs. 43.3(11.9) g/m2.7, p < 0.0001], left atrium volume [25.7(2.5) vs. 25.0(2.8) ml/m2, p = 0.003], and aortic root diameter [33.6(3.0) vs. 33.0(3.7) mm, p < 0.0001]. They did not differ for microalbuminuria and estimated glomerular filtration rate. At multivariate analysis, after adjustment for office systolic blood pressure values, the STOP-Bang score remained a predictor of left ventricular mass index; while the Insomnia Severity Index and restless legs syndrome risk score had no predictive value. However, a significant interaction between STOP-Bang and Restless Legs Syndrome Rating Scale scores in determining left ventricular remodelling was found.
Conclusions: In consecutive hypertensive stage I patients the STOP-Bang questionnaire allowed identification of a high-risk cohort featuring a more prominent cardiac damage. Hence, this inexpensive tool can be useful for risk stratification purposes in municipalities with limited access to health care resources.
Introduction
Arterial hypertension (HT), the most common cardiovascular (CV) risk factor worldwide, caused 10·4 million deaths and up to 54% of all strokes and 49% of cardiac attacks only in 2013 [Citation1]. In hypertensive patients the onset of CV events is preceded by a long asymptomatic phase during which hypertension-mediated organ damage (HMOD) develops. Hence, as identification of subclinical HMOD is useful for risk stratification purposes [Citation2], available guidelines recommend the systematic assessment of subclinical HMOD [Citation3,Citation4]. In clinical practice glomerular filtration rate (GFR) [Citation5], 24-hour urinary albumin excretion (UAE) [Citation6], and left ventricular mass index (LVMI), which were shown to predict CV events in all ethnic groups of hypertensive patients [Citation6], are, therefore, measured [Citation2,Citation3,Citation7].
In hypertensive patients chronic sleep deprivation and decreased sleep quality are emerging health problems [Citation7], which might contribute to worsening to HT and its complications [Citation7,Citation8]. The three most common sleep disorders in adults entail insomnia, restless legs syndrome (RLS), and obstructive sleep apnoea (OSA) [Citation7]. Insomnia has a prevalence in the general population of 5 – 15% [Citation9]; furthermore, it raises by 4-fold the risk of developing HT and CV complications, in particular if associated with short sleep duration (< 4 – 6 hours of sleep per night) and hyperarousals [Citation7,Citation10]. The RLS (Willis–Ekbom Disease), a common (5 – 9%) sensorimotor disorder associated with HT, especially in women and in chronic kidney disease and/or diabetes mellitus patients, also impairs sleep quality [Citation11,Citation12], and might contribute to CV diseases, particularly when related to periodic limb movements [Citation11,Citation12].
Accumulating evidences also suggest an association of OSA and HT, CV events, and metabolic dysfunction exists [Citation13,Citation14]. This is most relevant in that moderate-to-severe OSA (apnoea/hypopnea index - AHI ≥ 15 events per hour) involves up to 30% of patients with HT [Citation15]. The apnoeic events occurring in night-time are held to trigger sympathetic bursts that affect heart and vessels [Citation16], which might explain why moderate-to-severe OSA patients develop more concentric left ventricular hypertrophy (LVH) [Citation16–18] and renal damage than non OSA or mild OSA hypertensive patients [Citation19]. Moreover, effective treatment of OSA with continuous positive airway pressure (CPAP) was reported to lower blood pressure (BP), and to regress concentric LVH in patients with HT and recurrent atrial fibrillation [Citation20,Citation21].
As insomnia, OSA, and RLS often co-exist in individual patients [Citation7], but their association and interaction with HMOD is unknown, we set out this study to prospectively investigate the hypothesis that these sleep disorders, as ascertained by means of self-reported validated questionnaires, can allow identification of hypertensive patients with higher risk of carrying HMOD.
Materials and methods
We consecutively recruited hypertensive patients of both gender, referred to our ESH Excellence Hypertension Centre of University of Padua, Italy, following the flow-chart shown in Figure S1 of Supplemental Data. The diagnosis of HT was made in patients with a previous diagnosis of HT on treatment with antihypertensive drugs, and/or if systolic BP (SBP) was ≥ 140 mmHg and diastolic BP (DBP) ≥ 90 mmHg [Citation3] at three consecutive measurements taken 5 minutes apart by the same investigator. Office BP was measured with an automated device following the ESC/ESH guidelines [Citation3]. Exclusion criteria were: (i) known/treated sleep disorders, (ii) night shift workers, (iii) treatment with drugs influencing central nervous system, (iv) alcohol and/or drugs abuse, (v) pregnancy.
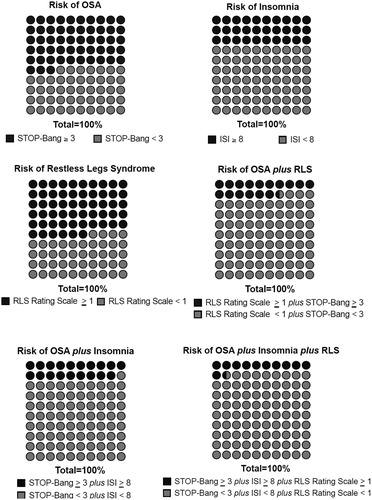
Figure 1. Prevalence of pathological score at the administered questionnaires in our population. ISI: Insomnia Severity Index; RLS: restless legs syndrome.
After the baseline clinical assessment, which included past medical history, current treatment, and detailed information about CV risk (i.e. smoking, body mass index – BMI, etc.), all subjects underwent physical examination, biochemical evaluation, including serum creatinine and estimated GFR (eGFR by Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [Citation22]), and screening for secondary forms of HT as reported [Citation3].
Questionnaires
After signing an informed consent, all patients were administered validated questionnaires to evaluate the risk of insomnia, OSA, and RLS (please see Supplemental Data for details). The Insomnia Severity Index (ISI), was scored as follows: absence of insomnia (0 – 7); sub-threshold insomnia (8 – 14); moderate insomnia (15 – 21); and severe insomnia (22 – 28) [Citation23].
The Snoring Tiredness Observed apnoea high BP - BMI Age Neck circumference Gender (STOP-Bang) score estimates the risk of OSA and ranges from 0 to 8 [Citation24]. We use the STOP-Bang score with a cut-off ≥ 3 instead of a newer score [Citation25], because it was the only one validated at the time when our study was conceived and submitted to Ethical Committee. With a cut-off score ≥ 3, in fact, this questionnaire demonstrated a sensitivity of 84% in detecting any sleep apnoea (AHI > 5 events/h), of 93% in moderate-to-severe form (AHI > 15 events/h), and 100% sensitivity for severe sleep apnoea (AHI > 30 events/h) [Citation24].
Moreover, compared with other sleep apnoea screening questionnaires, as the Berlin questionnaire [Citation26] and OSA50 [Citation27], the STOP-Bang showed the best likelihood negative ratio for the prediction of OSA in obese patients [Citation28], indicating that it represents a simple, effective, and reliable screening tool for diagnosing and treating previously unrecognized OSA.
To assess RLS severity we used the RLS Rating Scale, which rules out RLS if the score is 0 [Citation29].
Finally, to evaluate the impact of sleep disorders on excessive daytime sleepiness, we used the Epworth Sleepiness Scale (ESS), where a score ≥ 10 indicates high risk of sleepiness [Citation30].
Echocardiography
M-mode and 2D echocardiography (Vivid 7 Pro®, General Electric) was performed following available guidelines as reported in detail [Citation31] and summarised in the Supplemental Data.
GFR and UAE rate
GFR was measured by using the CKD-EPI equation, which takes into consideration serum creatinine, age, gender, race, and body surface area [Citation22]: estimated GFR (mL/min/1.73 m2) = 141 × min(SCr/κ, 1)α × max(SCr/κ, 1)−1.209 × 0.993Age × 1.018 [if female] × 1.159 [if Black] [Citation22].
Urinary albumin and creatinine excretion was measured with commercially available radioimmunoassay (H ALB kit-double antibody; Sclavo SpA) or immunoturbidimetry assay kit (Sera-Pak, Bayer). UAE rate was analysed as milligrams × 24 hours−1 and also after normalization for milligrams of urinary creatinine. The normal range of UAE rate was < 30 mg × g−1 of creatinine (or < 30 mg × 24 hours−1).
Statistical analysis
For descriptive purposes continuous variables were expressed as mean and standard deviation (SD) (or SEM), or median and interquartile range (IQR), as appropriate. Before statistical analysis, variables that showed a non-Gaussian distribution at Kolmogorov-Smirnov test were transformed to achieve a normal distribution; if a normal distribution could not be achieved, they were analysed by non-parametric tests. Continuous variables were compared between different subgroups (STOP-Bang < 3 vs. STOP-Bang ≥ 3, ISI < 8 vs. ISI ≥ 8, and RLS Rating Scale = 0 vs. ≥ 1) by the Student’s t-test, or Mann-Whitney test. Categorical variables were compared with Fisher and chi square tests. The Fisher's Least Significant Difference Post-Hoc test was used to detect differences between groups when ANOVA showed significant differences across them. Linear regression models and multivariate analysis were performed to determine the combined effect of several variables on HMOD markers. A further multiple regression model was undertaken to investigate a possible interaction between STOP-Bang and RLS Rating Scale score on LVMI. To avoid issues of collinearity, we calculated the centered mean value of both STOP-Bang and RLS Rating Scale and then subtracted it from the absolute values of the scores. An interaction term was calculated by multiplying the mean-subtracted STOP-Bang and RLS Rating Scale values. This interaction term was entered in the regression model (please see Supplemental Data for the SPSS Syntax algorithm, Figure S2).
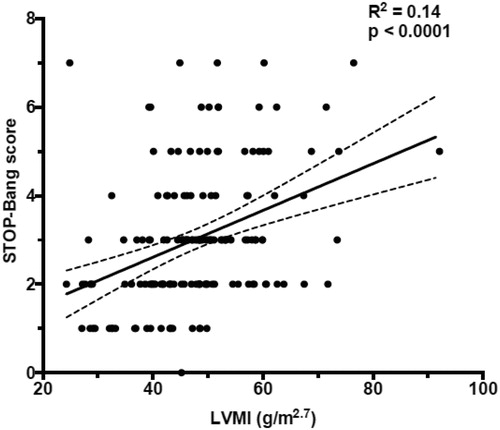
Figure 2. Bivariate analysis using scatter plot and Pearson's correlation test to assess the correlation between STOP-Bang score and left ventricular mass index (LVMI).
Statistical analysis was performed using SPSS software (version 24 for Mac; IBM®, SPSS® Statistics, Italy) and GraphPad Prism software (version 8.0, GraphPad® Software Inc, San Diego, CA, USA). Significance was set at P < 0.05.
Results
Anthropometric, biochemical, and echocardiographic features
We recruited 159 consecutive referred hypertensive patients [(98 men, 61 women, age 47 (11) years, median and (IQR)]. A thorough diagnostic work-up performed after washout from drugs affecting plasma aldosterone concentration and active renin, allowed to conclusively rule out secondary forms of HT in 105 patients (65.4%), who were therefore considered to have primary (essential) HT; secondary forms of hypertension were found in 34.6%, mostly (25.6%) primary aldosteronism. and Citation2 show the main features of these patients, including echocardiographic and Doppler characteristics. A STOP-Bang score ≥ 3 was found in 52.8% of the patients, mostly men. 21.1% of these patients had primary aldosteronism; an ISI score ≥ 8 was observed in 30.2% with no between-sexes differences; 56% of patients showed an RLS Rating Scale > 0 (34% females) ().
Table 1. Baseline demographic and anthropometric data.
Compared to patients with a STOP-Bang score < 3, those with an OSA ≥ 3, i.e. at high risk of OSA, were older, overweight, and required more antihypertensive drugs to achieve BP target values. They also showed higher aldosterone/renin ratio (ARR) and salt intake ().
All subgroups showed a normal renal function, but, serum creatinine levels were higher in the high compared to the low STOP-Bang score patients [78 (16) vs. 74 (21) μmol/l, p = 0.03], and in the high STOP-Bang than in the high ISI score [78 (16) vs. 73 (20) μmol/l, p = 0.03]. At variance, eGFR and UAE did not differ significantly across subgroups ().
Table 2. Baseline biochemical and echocardiographic data.
Table 3. Demographic and biochemical data in the subgroups with and without high risk of OSA, insomnia, and RLS, based on the relative questionnaire scores.
The 24-hour urinary excretion of free catecholamines (norepinephrine and epinephrine) and their metabolites (normetanephrine and metanephrine), a proxy of sympathetic nervous system (SNS) activity, showed an increased norepinephrine excretion [375 (270) vs. 326 (210) µmol/24h, p = 0.018] in high vs. low OSA risk (STOP-Bang ≥ 3 vs. < 3) (Table S2 – Supplemental Data). No such differences were seen between subgroups of patients split according to ISI (8 ≥ vs. < 8) and RLS Rating Scale scores (≥ 1 vs. 0). By examining the impact of sleep disorders on excessive daytime sleepiness we discovered that any subgroup of patients had ESS pathological score (≥ 10) (Supplemental Data, Table S3).
Relationships between hypertensive-mediated organ damage and STOP-Bang score
A multivariate scatter plot exploratory analysis showed a direct correlation of LVMI with the STOP-Bang, but not with the other sleep disorders scores, which was confirmed at bivariate scatterplot and correlation analysis of the STOP-Bang score with LVMI (r = 0.37, p < 0.0001) (), left atrium (LA) size (LA volume index – LAVI) (r = 0.22, p = 0.004), aortic root dimension (r = 0.36, p < 0.0001), E/e’ (r = 0.27, p < 0.0001), and E/A ratio (r = −0.22, p = 0.005).
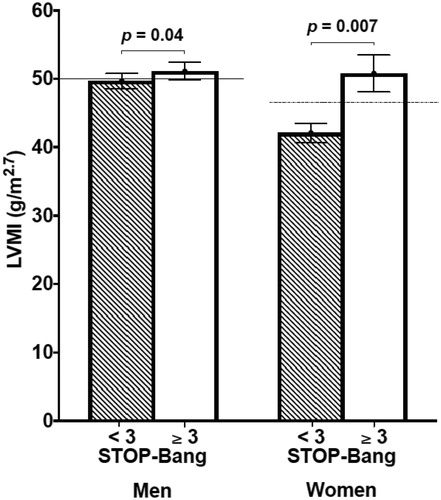
Thus, patients with high OSA risk score showed higher LVMI values () than those with low OSA risk score and also than patients with RLS Rating Scale ≥ 1. Only a trend toward a higher rate of LVH (59.5% vs. 53.3%, p = 0.25) in high vs. low STOP-Bang score patients was seen. A sensitivity analysis performed by gender, using the gender-specific LVMI cut-offs recommended by the last ESC/ESH hypertension guidelines [Citation3] confirmed these findings ().
Figure 3. The bar graph shows the left ventricular mass index (LVMI, g/m2.7) in the different subgroups obtained from the three questionnaires scores.
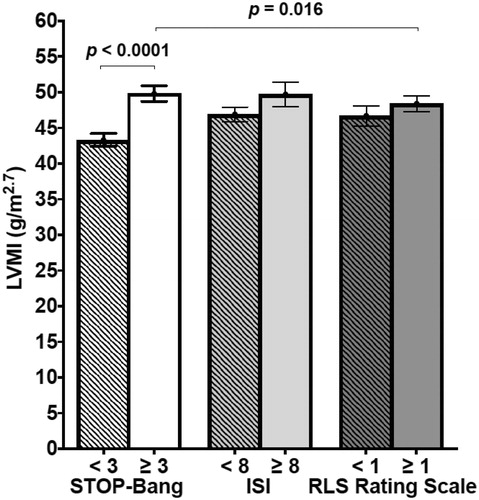
Figure 4. The bar graph shows the left ventricular mass index (LVMI) in patients with low risk of OSA (STOP-Bang < 3) compared with high risk (STOP-Bang ≥ 3) divided by gender (number of men = 31 vs. 67, number of women 44 vs. 17, respectively). The continuous line indicates the normal value of LVMI in males (< 50 g/m2.7); the dashed line the normal value of LVMI in females (< 47 g/m2.7).
Noteworthy, compared to the low STOP-Bang score group, the high OSA risk patients also demonstrated higher LAVI [25.7 (2.5) vs. 25.0 (2.8) ml/m2, p = 0.003] and aortic root dimension [33.6 (3.0) vs. 33.0 (3.7) mm, p < 0.0001]. These findings were confirmed at sensitivity sub-analyses, considering essential hypertensive patients and the secondary forms of hypertension, mainly represented by primary aldosteronism ().
Table 4. Main results from analysis for type of hypertension.
On a multivariate analysis, the strongest predictors of LVMI were the STOP-Bang score and office SBP, a finding confirmed at a further sensitivity analysis of the cohorts of patients with primary (β = 0.254, p = 0.007) and secondary HT (β = 0.462, p = 0.005). The STOP-Bang score was associated also with aortic root diameter, independently from age, office SBP and DBP values (β = 0.44, p = 0.012). Moreover, a further multivariate regression analysis allowed identification of a significant interaction between STOP-Bang and RLS Rating Scale scores on determining LVMI values (Supplemental Data). Of note, the correlation coefficients showed a positive value for centered STOP-Bang (β = 0.296, p < 0.0001) and a negative value for the associated variable between STOP-Bang and RLS Rating Scale (β = −0.184, p = 0.012), indicating that the latter is a modulator of the effect of high OSA risk patients on LVMI ().
Table 5. Multiple regression analysis.
Relationships between hypertensive-mediated organ damage, ISI, and RLS Rating Scale
By similar approaches we could find no evidence for a relationship between HMOD markers and high scores at the ISI and RLS Rating Scale questionnaires at bivariate scatterplot and correlation analysis, and by comparing patients with high vs. low risk of RLS, e.g. an RLS Rating Scale score ≥ 1 and insomnia (ISI score ≥ 8). To further challenge these findings, we performed a sensitivity analysis using higher cut-off scores, defining a mild form of disease, for both the questionnaires (ISI > 14 and RLS Rating Scale > 10). This confirmed the lack of differences in the anthropometric, clinical data, and comorbidities between groups, with the exception of a higher eGFR and a lower UAE in those with an ISI ≤ 14 vs. those with ISI > 14 (Supplemental Data, Table S4), indicating a better renal function in patients with low insomnia score. Also for the subgroup with RLS Rating Scale ≤ 10 the UAE was lower compared to patients with RLS Rating Scale > 10 (Supplemental Data, Table S4). The latter difference waned when serum creatinine-adjusted UAE was examined [Citation32].
Finally, to address the question if the subgroup with concurrent high risk of OSA, insomnia, and RLS could show distinctive features in terms of HMOD, we examined the cohort with the highest (pathological) scores in all 3 questionnaires (STOP-Bang ≥ 3 plus RLS Rating Scale score ≥ 1 plus ISI ≥ 8), which entailed only 18 (11.3%) of our patients. Of interest, patients in this subgroup were older [50 (6) vs. 46 (11) years, p = 0.01], and heavier [BMI 28.5 (7.9) vs. 24.9 (5.8) kg/m2, p = 0.005], and showed higher LVMI [52.7 (11.0) vs. 46.9 (11.6) g/m2.7, p = 0.004], and increased LAVI [26.4 (3.2) vs. 25.7 (4.0) mm, p = 0.046].
Discussion
By investigating, prospectively, the prevalence of the three most common disorders of sleep by means of simple validated and reproducible questionnaires, we found that they were frequent in patients referred to a tertiary centre for hypertension: 18.9% had both OSA and insomnia, 28.9% a high risk of OSA and RLS, while fewer patients (11.3%) had high scores for all three sleep disorders (). These questionnaires are simple, inexpensive, fast, and easy to implement for routine clinical use. Hence, we would like to contend that investigation of sleep disorders with validated questionnaires should become part of the clinical work-up of patients with HT, at least in the selected cohort of patients referred to specialised HT centres.
The present discovery that such disorders have an impact on the clinical management of these patients supports this contention for the following reasons. When compared to the subgroup of patients with a low risk of OSA, those at increased risk (STOP-Bang score ≥ 3) exhibited more hypertension-related cardiac damage, including increased LVMI, left atrium dilatation, and aortic root enlargement (). Increased sympathetic activity, activation of the renin-angiotensin-aldosterone system, endothelial dysfunction, systemic inflammation, oxidative stress, and metabolic abnormalities are putative mechanisms that can explain OSA-related cardiac hypertrophy and fibrosis, altering the lusitropic properties of the LV, causing atrial dilatation, and ultimately impairing LV diastolic and systolic function [Citation16–18,Citation33]. Moreover, these changes can explain the well-documented increased risk of atrial fibrillation in OSA patients [Citation34].
It is worth noting that the STOP-Bang questionnaire predicted LVMI independently of age and plasma aldosterone in our cohort, suggesting that this questionnaire can be useful for risk stratification purposes. The finding of a significant interaction between patients with higher risk of OSA and RLS, with an opposite relationship with LVMI, is a further novel result of this study (Supplemental Data). This model suggests that, while OSA contributes to LVMI damage, RLS might have a modulator or protective effect, whose nature deserves specific further researches.
Currently, the reference standard for diagnosing OSA is an overnight polysomnography [Citation35], a time-consuming and costly procedure, whose availability is being even further restricted in most municipalities, because of an exponentially increased demand owing to the growing awareness of OSA and to the need of sleep medicine specialists. These factors may preclude a prompt diagnosis and an early treatment of OSA in the majority of the patients who need it. It is therefore important to underline that a simple, validated [Citation24] questionnaire for determining the risk of OSA was found to allow identification of cardiac damage in a sizable cohort of our referred consecutive hypertensive patients. This questionnaire, which has been proven to be accurate for the screening of OSA patients [Citation36], could, therefore, be useful for CV risk stratification purposes to pinpoint which hypertensive patients are more likely to have adverse cardiac remodelling and CV consequences.
At variance with the STOP-Bang, we found no evidence of an association of HMOD with insomnia and/or RLS questionnaires, a negative finding possibly due to the small sample size of our cohort and/or to the fact that these two sleep disorders have a less impact than OSA on BP values and HT-related target organ damage. Further, larger studies using wider sample size, more accurate information on the true sleep hours, and a more precise assessment of BP and on the dipping profile by means of 24-hour ambulatory BP monitoring are, however, necessary before insomnia and RLS questionnaires can be dismissed as valuable tools to detect HMOD in hypertensive patients [Citation3]. Unfortunately, we could perform the 24-hour ambulatory BP monitoring in a number of patients too small to furnish solid information on the role of circadian variation of BP.
As regards renal-hypertensive damage, we found no statistically significant differences between patients with high risk of OSA compared with low risk subgroup, although a signal for a better renal function in those with less insomnia was detected in a sensitivity analysis using a more restrictive cut-off for the insomnia score. Overall, these negative findings could be due to (i) the relative young population examined, (ii) the mild degree of BP elevation (grade I according to guidelines [Citation3]); (iii) the absence of malignant HT, and (iv) the longer time period for renal damage to develop as compared to cardiac remodelling [Citation37].
Notably, we found higher ARR values in high- than in low-risk OSA group, which could be mechanistically relevant inasmuch as aldosterone is implicated in the onset of HMOD [Citation38,Citation39]. Aldosterone can worsen OSA by promoting fluid accumulation, which in the supine position shifts to the neck, thus contributing to increased upper airway resistance [Citation40].
Additionally, our patients with STOP-Bang score ≥ 3 exhibited significantly higher 24-hour urinary norepinephrine excretion than those with high scores at the ISI questionnaire (Supplemental Data, Table S1), a finding consistent with the views that an altered autonomic nervous system drive is a feature of OSA [Citation41]. Recurrent episodes of partial and/or total airway collapse cause an increased SNS activity in OSA patients; moreover, treatment with CPAP effectively reduced this SNS overactivation [Citation42].
Considering the role of neurohormonal factors in cardiac remodelling, we would like to contend that the higher values of ARR, urinary norepinephrine excretion, along with sodium intake as assessed by 24-hour urinary sodium excretion, can be proxies for cardiac remodelling in high-risk of OSA patients. As all available hypertension guidelines emphasise the need for risk stratification of the patients [Citation1–4] by means of a complex and expensive work-up, we would like to propose that the STOP-Bang questionnaire, a simple instrument that takes only few minutes to be completed, can be used to select patients with higher CV risk patients.
Limitations and strengths
Some limitations to be acknowledged, besides the relatively limited sample size and the lack of 24-hour ambulatory blood pressure, include the lack of (i) data on sleep duration, which has been demonstrated to correlate with insomnia and higher incidence of hypertension when < 4 – 6 hours per night [Citation10,Citation43], (ii) of a full-night polysomnography, which was performed only in few patients because at the time when data collection started we could not perform such investigation systematically. Upon completion of this pilot study we have started performing these investigations in all patients with higher values of STOP-Bang and found that this questionnaire accurately identified the patients with severe OSA, in line with the results of Gami et al. [Citation34].
There are, however, major strengths to be underlined in this study, including its prospective design, use of validated questionnaires to screen sleep disorders and their consequences on daily activity and sleepiness, a thorough hormonal assessment, the measurement of sodium intake by a 24-hour urine collection, and systematic adoption of transthoracic echocardiography to assess cardiac remodelling.
Conclusions
In summary, we found that a STOP-Bang questionnaire score ≥ 3, a cut-off with high sensitivity and specificity, and recommended by the validation study [Citation24], was useful to identify hypertensive subjects with higher risk of hypertensive-induced cardiac remodelling. Moreover, the hypertensive patients with such high scores showed inappropriately high levels of ARR and urinary norepinephrine excretion in spite of a higher sodium intake. The importance of this finding relies in the fact that these patients might take particular benefit from an aggressive treatment, including target interventions, as lowering salt intake, mineralocorticoid receptor antagonists and/or α- plus β-adrenergic-receptor blockers. Therefore, the STOP-Bang questionnaire can be used as a screening tool for pinpointing hypertensive patients with a higher likelihood of carrying cardiac target organ damage in whom to concentrate a more intense diagnostic and therapeutic intervention.
supplemental_data_2019_jan_23.docx
Download MS Word (640.9 KB)Acknowledgements
The authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Olsen MH, Angell SY, Asma S, et al. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: the Lancet Commission on hypertension. Lancet. 2016;388:2665–2712.
- Sehestedt T, Jeppesen J, Hansen TW, et al. Thresholds for pulse wave velocity, urine albumin creatinine ratio and left ventricular mass index using SCORE, Framingham and ESH/ESC risk charts. J Hypertens. 2012;30:1928–1936.
- Williams B, Mancia G, Spiering W, et al. The task force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). 2018 ESC/ESH Guidelines for the management of arterial hypertension. J Hypertens. 2018;36:1953–2041.
- Flack JM, Calhoun D, Schiffrin EL. The new ACC/AHA hypertension guidelines for the prevention, detection, evaluation, and management of high blood pressure in adults. Am J Hypertens. 2018;31:133–135.
- Matsushita K, Mahmoodi BK, Woodward M, et al. Chronic kidney disease prognosis consortium. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA 2012;307:1941–1951.
- Stevens LA, Coresh J, Greene T, et al. Assessing kidney function-measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483.
- Tobaldini E, Costantino G, Solbiati M, et al. Sleep, sleep deprivation, autonomic nervous system and cardiovascular diseases. Neurosci Biobehav Rev. 2017;74:321–329.
- Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–839.
- American Academy of Sleep Medicine. The international classification of sleep disorders, 3th ed. Darien (IL): American Academy of Sleep Medicine; 2014.
- Thomas SJ, Calhoun D. Sleep, insomnia, and hypertension: current findings and future directions. J Am Soc Hypertens. 2017;11:122–129.
- Ferri R, Fulda S, Allen RP, et al. International and European Restless Legs Syndrome Study Groups (IRLSSG and EURLSSG). World Association of Sleep Medicine (WASM) 2016 standards for recording and scoring leg movements in polysomnograms developed by a joint task force from the International and the European Restless Legs Syndrome Study Groups (IRLSSG and EURLSSG). Sleep Med. 2016;26:86–95.
- Walters AS, Rye DB. Review of the relationship of restless legs syndrome and periodic limb movements in sleep to hypertension, heart disease, and stroke. Sleep. 2009;32:589–597.
- Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384.
- Somers VK, White DP, Amin R, et al. American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology; American Heart Association Stroke Council; American Heart Association Council on Cardiovascular Nursing; American College of Cardiology Foundation. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation. 2008;118:1080–1111.
- Floras JS. Hypertension and sleep apnea. Can J Cardiol. 2015;31:889–897.
- Konecny T, Somers VK. Sleep-disordered breathing in hypertrophic cardiomyopathy: challenges and opportunities. Chest. 2014;146:228–234.
- Baguet JP, Barone-Rochette G, Tamisier R, et al. Mechanisms of cardiac dysfunction in obstructive sleep apnea. Nat Rev Cardiol. 2012;9:679–688.
- Prejbisz A, Florczak E, Pręgowska-Chwała B, et al. Relationship between obstructive sleep apnea and markers of cardiovascular alterations in never-treated hypertensive patients. Hypertens Res. 2014;37:573–579.
- Tsioufis C, Thomopoulos C, Dimitriadis K, et al. Association of obstructive sleep apnea with urinary albumin excretion in essential hypertension: a cross-sectional study. Am J Kidney Dis. 2008;52:285–293.
- Haentjens P, Van Meerhaeghe A, Moscariello A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med. 2007;167:757–764.
- Koga S, Ikeda S, Nakata T, et al. Effects of nasal continuous positive airway pressure on left ventricular concentric hypertrophy in obstructive sleep apnea syndrome. Intern Med. 2012;51:2863–2868.
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612.
- Morin CM, Belleville G, Bélanger L, et al. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601–608.
- Chung F, Abdullah HR, Liao P. STOP-bang questionnaire: a practical approach to screen for obstructive sleep apnea. Chest. 2016;149:631–638.
- Marti-Soler H, Hirotsu C, Marques-Vidal P, et al. The NoSAS score for screening of sleep-disordered breathing: a derivation and validation study. Lancet Respir Med. 2016;4:742–748.
- Netzer NC, Stoohs RA, Netzer CM, et al. Using the Berlin questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–491.
- Chai-Coetzer CL, Antic NA, Rowland LS, et al. A simplified model of screening questionnaire and home monitoring for obstructive sleep apnoea in primary care. Thorax. 2011;66:213–219.
- Prasad KT, Sehgal IS, Agarwal R, et al. Assessing the likelihood of obstructive sleep apnea: a comparison of nine screening questionnaires. Sleep Breath. 2017;21:909–917.
- Walters AS, LeBrocq C, Dhar A, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4:121–132.
- Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea. The Epworth Sleepiness Scale. Chest. 1993;103:30–36.
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14.
- Rossi GP, Bernini G, Desideri G, et al. PAPY Study Participants. Renal damage in primary aldosteronism: results of the PAPY Study. Hypertension. 2006;48:232–238.
- Oliveira W, Campos O, Cintra F, et al. Impact of continuous positive airway pressure treatment on left atrial volume and function in patients with obstructive sleep apnoea assessed by real-time three-dimensional echocardiography. Heart. 2009;95:1872–1878.
- Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110:364–367.
- Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Adult obstructive sleep apnea task force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–276.
- Abrishami A, Khajehdehi A, Chung F. A systematic review of screening questionnaires for obstructive sleep apnea. Can J Anesth. 2010;57:423–438.
- Mennuni S, Rubattu S, Pierelli G, et al. Hypertension and kidneys: unraveling complex molecular mechanisms underlying hypertensive renal damage. J Hum Hypertens. 2014;28:74–79.
- Monticone S, D'Ascenzo F, Moretti C, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018;6:41–50.
- Rossi GP, Maiolino G, Flego A, et al. PAPY study investigators. Adrenalectomy lowers incident atrial fibrillation in primary aldosteronism patients at long term. Hypertension. 2018;71:585–591.
- Wolley MJ, Pimenta E, Calhoun D, et al. Treatment of primary aldosteronism is associated with a reduction in the severity of obstructive sleep apnoea. J Hum Hypertens. 2017;31:561–567.
- Somers VK, Dyken ME, Mark AL, et al. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med. 1993;328:303–307.
- Dimsdale JE, Coy T, Ziegler MG, et al. The effect of sleep apnea on plasma and urinary catecholamines. Sleep. 1995;18:377–381.
- Fernandez-Mendoza J, Vgontzas AN, Liao D, et al. Insomnia with objective short sleep duration and incident hypertension: the Penn State Cohort. Hypertension. 2012;60:929–935.
