Abstract
Objective: Advanced technology allows non-invasive monitoring of the 24-h brachial and central hemodynamics simultaneously. However, related reproducibility data was limited in White patients. We therefore explored if the novel measurements would be reproducible in Chinese.
Methods: From February 2017 to January 2018, 152 untreated patients who were suspected of hypertension and referred for ambulatory blood pressure (BP) monitoring were recruited. Ambulatory BP monitoring was repeated within one month (median, 12.5 days) using the Mobil-O-Graph monitors (IEM, Germany). Reproducibility was assessed as the intra-class correlation coefficient (ICC), coefficient of variation (CV), and repeatability coefficient (RC).
Results: The 152 participants (average age, 58.6 years) included 54 men and 98 women. The first and second means of the ambulatory brachial and central BPs, pulse wave velocity (PWV), augmentation pressure, augmentation index (AIx) and AIx at a heart rate of 75 beats per minute (AIx@75) were all similar (p ≥ 0.065), except that the repeated daytime and 24-h brachial and central systolic BPs and pulse pressure slightly differed by approximately 1-2 mmHg (p ≤ 0.011). ICC ranged from 0.70 to 0.94 for all ambulatory BPs and ≥0.91 for the arterial measurements. CV was in the range from 5.0% to 10.3% for all BPs and PWV measurements, and from 15.5% to 22.3% for AIx and AIx@75. RC expressed as percentages of maximal variation was <15% for the PWVs and ranged from 25.5% to 54.7% for BPs, AIx and AIx@75.
Conclusions: The 24-h ambulatory brachial and central BPs and arterial measurements were reproducible within a short time period in Chinese, and could therefore be used in clinical practice and research settings.
Introduction
Ambulatory blood pressure (BP) monitoring is increasingly used in clinical practice for hypertension management, because it compared with office measurement contains more BP information, and is more reproducible [Citation1] and more closely related to target organ damage [Citation2,Citation3] and cardiovascular outcome [Citation4–6]. Advanced technology in pulse wave analysis extends the noninvasive ambulatory BP monitoring from the brachial artery to the central artery, and provides indirect assessment of arterial stiffness and wave reflections simultaneously [Citation7–9].
In 2010, Wassertheurer and colleagues introduced a novel brachial cuff-based oscillometric device which could not only monitor brachial BP, but also record brachial pulse wave forms during BP measurement, and estimate central BP, aortic pulse wave velocity and wave reflections [Citation7]. Although the inbuilt ARCSolver algorithm has been validated against invasive and noninvasive references for its accuracy in the estimation of brachial and central BPs [Citation10–12] and arterial parameters [Citation13,Citation14], the reproducibility data was only limited to a study in 30 White patients untreated or treated with antihypertensive medications [Citation15,Citation16]. In view of the more and more widely use of this novel technology in Chinese and the disparity in central hemodynamics between ethnicities [Citation17], our study aimed to explore if the measurements derived from this novel device would be reproducible in Chinese patients free of the influence of antihypertensive medications.
Methods
Study population
In the framework of an ongoing project on the cardiovascular health of outpatients, we recruited consecutive untreated adults (≥30 years) referred for ambulatory BP monitoring to the Hypertension Outpatient clinic of Ruijin Hospital, Shanghai, China, as described previously [Citation18]. In brief, outpatients who were uncertain about their BP status irrespective of antihypertensive treatment were referred by physicians to the clinic for the 24-h ambulatory BP monitoring. If patients aged ≥30 years and were never treated (∼90%) or discontinued antihypertensive drugs for at least 2 weeks (∼10%), they were invited to participate in the study without any payment. We adhere to the principles of the Declaration of Helsinki, and the study protocol was approved by the Ethics Committee of Ruijin Hospital, Shanghai Jiaotong University School of Medicine. All patients gave informed written consent.
Of those referred between February 2017 to January 2018, 171 subjects were eligible for inclusion in the current analysis, because they had repeated 24-h ambulatory BP monitoring within one month. From the analysis, we excluded 18 subjects because they had unsuccessful ambulatory BP monitoring at least once (less than 20 hours of duration or less than 20 daytime or 7 nighttime brachial BP readings of a recording [Citation19]), and 1 subject because she initiated antihypertensive drug treatment between the two recordings. Finally, 152 participants were included for analysis.
Ambulatory brachial and central hemodynamics monitoring
The 24-h ambulatory monitoring of brachial and central hemodynamics was performed twice within one month. We programmed the oscillometric Mobil-O-Graph PWA monitors (IEM, Stolberg, Germany) to obtain BP and arterial measurements every 20 minutes from 6:00 to 22:00 and every 30 minutes from 22:00 to 6:00. Participants were requested to record a diary on the time of going to sleep at night and awakening in the morning during the monitoring. The daytime and nighttime was defined according to the diary.
The Mobil-O-Graph PWA monitors first perform brachial BP measurement, and immediately afterwards record brachial pulse waves when the brachial cuff is inflated to the diastolic BP level and held for about 10 seconds. Via a general transfer function, central pulse waves are generated, and central systolic BP, augmentation pressure, and augmentation index (AIx) unadjusted or adjusted at a heart rate of 75 beats per minute (AIx@75) are calculated by running the Mobil-O-Graph (HMS version 4.8) software with an inbuilt ARCSolver algorithm (Austrian Institute of Technology, Vienna, Austria) [Citation10]. The aortic pulse wave velocity (PWV) is also estimated by the ARCSolver algorithm with a mathematical model which utilizes age, central BP, aortic characteristic impedance and several parameters from pulse wave analysis and wave separation analysis [Citation13]. The quality of the pulse waves is graded by the inbuilt algorithm from 1 to 4 as excellent, good, moderate and poor, respectively, according to the percentages (>80%, >50%, <50%, and missing) of the cardiac cycles during signal acquisition. In the present analysis, estimations were used only if the signals were graded as 1 or 2. Ambulatory hypertension was defined as a 24-h brachial BP of at least 130 mmHg systolic or 80 mmHg diastolic.
Other measurements
A standardized questionnaire was administered to collect information on medical history, lifestyle, smoking and alcohol intake, and use of medications. Briefly, trained technicians enquired about medical history of hypertension, diabetes, coronary heart disease, stroke and other concomitant diseases, and the use of any drugs during the most recent two weeks. Current smoking was defined as at least one cigarette per day for at least one month most recently. From the type (beer, wine, yellow aperitif, and hard liquor) and quantity of the alcoholic beverages used, we computed alcohol consumption in grams per week to categorize participants into those not regularly drinking alcohol (<5 grams per week) and those consuming alcohol [Citation20]. Venous blood samples were drawn after overnight fasting for the measurement of serum total cholesterol, triglycerides, glucose, creatinine and uric acid. Diabetes mellitus was defined as a fasting blood glucose of at least 7.0 mmol/L or the use of antidiabetic agents, or a self-reported physician’s diagnosis of diabetes. Estimated glomerular filtration rate (eGFR) was calculated by the CKD-EPI creatinine equations [Citation21]. Each patient collected a first morning urine. Microalbuminuria was defined as an albumin-to-creatinine ratio of at least 3.4 milligrams per millimole [Citation22].
Statistical analysis
For database management and statistical analyses, we used SAS software, version 9.3 (SAS Institute Inc., Cary, North Carolina, USA). Because of the sex-difference in anthropometric characteristics and arterial wave reflection measurements [Citation23], we performed analysis separately in men and women as appropriate. Means and proportions between two independent groups were compared using the unpaired Student t-test and chi-square test, respectively, and means of repeated measurements using the paired t-test. Reproducibility was quantified using the following indices: standard deviation (SD) of the difference between repeated measurements; intra-class correlation coefficient (ICC); coefficient of variation (CV) that is the SD of the difference divided by the mean of the repeated measurements; repeatability coefficient (RC) that is twice the SD of the difference, or expressed as a percentage of 4 times the SD of the repeated measurements which is considered as close to maximal variation (pMV) [Citation24,Citation25]; and Bland-Altman plots [Citation26]. ICC values of 0.5–0.6 indicate moderate, 0.7–0.8 strong and >0.8 perfect agreement [Citation16]. Stepwise multivariate regression analysis was performed to identify the factors associated with the significant differences of the repeated BP measurements. A probability value of <.05 was considered statistically significant.
Results
Characteristics of the participants
The 152 participants included 54 men and 98 women, among whom 75 (49.3%) had a 24-h brachial BP of at least 130/80 mmHg, and 3 (2.0%) had an eGFR less than 60 ml/min*1.73 m2. Age averaged 58.6 ± 12.4 years. The median interval between the repeated 24-h ambulatory recordings was 12.5 days (range, 3 to 30 days). The first and second 24-h systolic/diastolic BPs averaged 125.7/79.2 mmHg at the brachial artery, and 117.3/80.7 mmHg at the central aorta.
lists the characteristics of the study participants by sex. Compared to men, women had smaller body size, less frequently reported cigarette smoking and alcohol drinking, and had a lower 24-h brachial diastolic BP, similar 24-h brachial and central systolic BPs, and thus higher 24-h brachial and central pulse pressures. Men and women had similar fasting blood glucose, serum triglycerides and eGFR, and a similar proportion of patients with microalbuminuria, but women had higher serum total cholesterol and urinary albumin-to-creatinine ratio. For arterial measurements, men and women had similar 24-h PWV (8.2 vs 8.6 m/s, p = .175), but as expected, women had higher (p < .001) wave reflection indices than men, including augmentation pressure (12.3 vs 7.5 mmHg), AIx (30.0% vs 21.0%) and AIx@75 (28.1% vs 19.8%).
Table 1. Characteristics of the study participants.
Quality control of the 24-h ambulatory monitoring
For all the 304 ambulatory recordings, the median (5th to 95th percentile interval) number of readings averaged to estimate the 24-h, daytime and nighttime brachial BPs were 62 (52–73), 29 (22–36), and 12 (11–12), respectively. The corresponding values were respectively 53 (34–64), 26 (16–33), and 9 (3–12) for central BPs as well as central arterial measurements. Detailed information on the quality of the first and second 24-h ambulatory measurements were provided respectively in Supplementary Tables S1 and S2.
Difference between the repeated measurements
shows that the first means of the ambulatory brachial and central BPs, PWV, AIx and AIx@75 were all similar (p ≥ 0.065) to the second means, except that the daytime and 24-h brachial and central systolic BPs and pulse pressures slightly differed (p ≤ 0.013) by approximately 1-2 mmHg, which was especially true in women (). In the 152 subjects, 44.7/70.4%, 36.2/21.7% and 19.1/7.9% had an absolute paired difference of ≤5 mmHg, 5.1–10 mmHg and >10 mmHg in brachial systolic/diastolic BPs, respectively, and 50.0/68.4%, 27.6/23.0%, 22.4/8.6% in central systolic/diastolic BPs (Supplementary Table S3).
Figure 1. Changes in the individual values (circle) and means ± SD (short horizontal line) from the first to the second measurements of the 24-h brachial (A) and central systolic blood pressure (SBP, B), pulse wave velocity (PWV, C) and augmentation index at a heart rate of 75 beats per minute (AIx@75, D) in 54 men and 98 women. The difference (Δ) of the second minus the first means and p values for the comparisons are given above the graph.
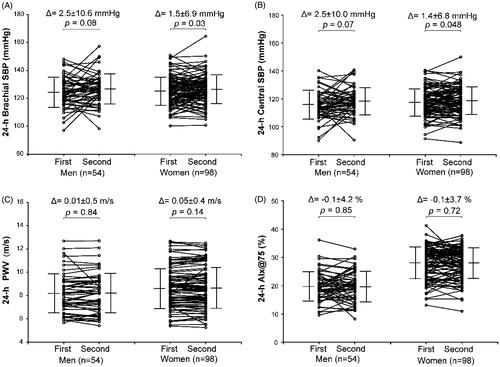
Table 2. Mean (±SD) of the first and second measurements of the 24-h brachial and central ambulatory blood pressures and arterial indices.
Supplementary Figure S1 shows the Bland-Altman plots for the 24-h brachial and central systolic BPs, PWV and AIx@75. There were no significant associations (p ≥ 0.511) between the difference and the mean of the repeated measurements. Multivariate stepwise regression analysis (Supplementary Table S4) revealed that the significant paired differences in the 24-h and daytime systolic BPs, irrespective of at brachial or central arteries, were all significantly associated with the time interval between the recordings (p ≤ 0.025) and cigarette smoking (p ≤ 0.002). For the 24-h and daytime central systolic BPs, the paired difference was additionally associated with the log-transformed triglycerides (p = .025) and body mass index (p = .041), respectively, and for the 24-h central pulse pressure, exclusively with the log-transformed triglycerides (p = .0069, Supplementary Table S4).
Reproducibility indices of the measurements
gives the reproducibility indices of the studied ambulatory BPs and arterial measurements, including the ICC and 95% confidence intervals, CV, and RC expressed as the 2 times of the SD of the paired difference or as the pMV. ICC ranged from 0.70 to 0.94 for all ambulatory BPs and ≥0.91 for PWV, augmentation pressure, AIx and AIx@75. CV was in the range from 5.0% to 10.3% for all BPs and PWV measurements, and from 15.5% to 22.3% for augmentation pressure, AIx and AIx@75. RC expressed as pMV was <15% for the PWV measurements and ranged from 25.5% to 54.7% for ambulatory BPs, augmentation pressure, AIx and AIx@75. Sensitivity analysis showed that the reproducibility of ambulatory BPs and arterial measurements was generally similar between subjects above or below the median age (59.9 years, Supplementary Table S5).
Table 3. Reproducibility indices of the ambulatory measurements.
Discussion
The key finding of our current study was that in Chinese patients free of antihypertensive medication the non-invasive 24-h brachial and central BPs and arterial measurements were reproducible within one month by using a cuff-based oscillometric device.
To the best of our knowledge, only two reports from one study in White patients addressed the reproducibility issue of the 24-h non-invasive brachial and central hemodynamics monitoring [Citation15,Citation16]. In 30 treated and untreated hypertensive patients with an average age of 53.6 years, 24-h ambulatory monitoring was performed with at least 1-week interval with the Mobilo-O-Graph monitors [Citation15,Citation16]. No significant differences in the average 24-h aortic BP values were observed between the two repeated assessments (115.9/79.7 vs. 115.1/79.2 mmHg, P ≥ 0.48). The reproducibility indices of aortic pressure, including the ICC (0.80–0.92) and SD of systolic/diastolic differences (6.0/4.5 mmHg), indicated acceptable reproducibility [Citation15]. Among the same 30 hypertensive patients, Papaioannou and colleagues examined the reproducibility of the 24-h measurements of aortic wave reflection and arterial stiffness [Citation16]. Similar to our observations, the aortic PWV, AIx and AIx@75 were highly reproducible with an ICC of >0.8. There were no systemic differences in the Bland-Altman plots and the limits of agreement indicated high reproducibility [Citation16].
As ethnicity is an important determinant for the central hemodynamic measurements [Citation17], it is necessary to confirm the reproducibility of the measurements in Chinese patients. In our study, in terms of the RC values, we observed that brachial and central BPs were similarly reproducible, whereas ambulatory BPs were less reproducible than the ambulatory arterial measurements. These observations were consistent with the above-mentioned first validation study using the same Mobil-O-Graph device [Citation15,Citation16]. The SDs of the paired differences were 6.3/4.5 mmHg and 6.9/4.9 mmHg, respectively, for the 24-h brachial and central systolic/diastolic BPs, indicating that brachial and central BPs were similarly reproducible within a short time period [Citation15]. If calculated on the basis of the reported SDs of the differences and the repeated means [Citation15,Citation16], the RCs expressed as pMV were around 30–40% for ambulatory BPs, but only 3% for PWV and approximately 20% for AIx and AIx@75, which were also quite similar to our findings.
Ambulatory brachial BP monitoring is increasingly used in the clinical practice of hypertension management as recommended by current guidelines [Citation22,Citation27]. Previous studies showed that the short-term reproducibility of the ambulatory brachial BP was moderate [Citation28–30], but better than the office BP [Citation1]. Our observations that the RCs of the 24-h, daytime and nighttime BPs ranged from 30 to 50% were consistent with the previous reports [Citation29,Citation30]. In 173 older (≥60 years) patients with isolated systolic hypertension who participated in the Syst-Eur trial [Citation29], repeated 24-h ambulatory recordings were performed within 33 days (median) with various validated monitors during the run-in period of the trial. The RCs expressed as pMV were 35%/42% for the daytime systolic/diastolic BPs, and 41%/41% for the nighttime BPs.
Several factors influence the accuracy and repeatability of ambulatory BPs, and therefore may explain the discrepancy between studies, such as sex [Citation31], changes in body weight [Citation32], effect of hospital environment [Citation32], physical activity during monitoring [Citation33], time interval between the recordings [Citation34], and the concomitant diseases, such as atrial fibrillation [Citation35]. In our study, the difference between two repeated ambulatory BPs was associated with the time interval between the recordings and smoking habit. The longer the interval was, the greater difference in BPs was observed, which was also true for the long-term reproducibility of ambulatory BP phenotypes [Citation34]. Cigarette smoking, as an important determinant of blood pressure variability during the day [Citation36], might influence the reproducibility of ambulatory BPs because of the difference in the number of cigarettes and time of smoking on two monitoring days.
Our study must be interpreted within the context of its potential limitations and strengths. First, the study subjects did not take any antihypertensive medications during the study, which may rule out influence of possible drug changes on the results. Second, we stuck to the recommendations [Citation19] and reported the quality of the ambulatory measurements in detail. However, current results may not be directly generalized to other populations, such as obese patients or patients with atrial fibrillation [Citation35]. In addition, we did not investigate the accuracy of the inbuilt algorithm of the device in the estimation of central BPs, PWV and wave reflections, although which has been validated in White populations, ethnicity, body mass index and heart rate are indeed important determinants of central hemodynamics [Citation37].
In conclusion, the short-term reproducibility of the cuff-based, oscillometric 24-h brachial and central hemodynamics monitoring was acceptable in Chinese. Our study provides further support for the feasibility of applying this new technique in clinical and research settings. On the other hand, further research is warranted to show the added value of the 24-h ambulatory to office central hemodynamic measurements and of the central to brachial measurements.
CBPM_reproducibility_spl_20190412.docx
Download MS Word (123.3 KB)Acknowledgements
The authors gratefully acknowledge the technical assistance of Junwei Li, Beiwen Lv, Jiaye Qian, Yuzhong Shi, Xiaokang Song, Qian Yu, Jie Zhou, Yi Zhou, Yini Zhou, and Jiajun Zong (Shanghai Institute of Hypertension, Shanghai).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Campbell P, Ghuman N, Wakefield D, et al. Long-term reproducibility of ambulatory blood pressure is superior to office blood pressure in the very elderly. J Hum Hypertens. 2010;24:749–754.
- Karpettas N, Destounis A, Kollias A, et al. Prediction of treatment-induced changes in target-organ damage using changes in clinic, home and ambulatory blood pressure. Hypertens Res. 2014;37:543–547.
- Angeli F, Reboldi G, Poltronieri C, et al. Clinical utility of ambulatory blood pressure monitoring in the management of hypertension. Expert Rev Cardiovasc Ther. 2014;12:623–634.
- Dolan E, Stanton A, Thijs L, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension. 2005;46:156–161.
- Hansen TW, Kikuya M, Thijs L, et al. Prognostic superiority of daytime ambulatory over conventional blood pressure in four populations: a meta-analysis of 7030 individuals. J Hypertens. 2007;25:1554–1564.
- Banegas JR, Ruilope LM, de la Sierra A, et al. Relationship between clinic and ambulatory blood-pressure measurements and mortality. N Engl J Med. 2018;378:1509–1520.
- Wassertheurer S, Kropf J, Weber T, et al. A new oscillometric method for pulse wave analysis: comparison with a common tonometric method. J Hum Hypertens. 2010;24:498–504.
- Brett SE, Guilcher A, Clapp B, et al. Estimating central systolic blood pressure during oscillometric determination of blood pressure: proof of concept and validation by comparison with intra-aortic pressure recording and arterial tonometry. Blood Press Monit. 2012;17:132–136.
- Krivoshei A, Lamp J, Min M, et al. Wearable system for non-invasive and continuous monitoring central aortic pressure curve and augmentation index. Stud Health Technol Inform. 2013;189:101–106.
- Weber T, Wassertheurer S, Mayer C, et al. Validation of a brachial cuff-based method for assessing central blood pressure. Hypertension. 2011;58:825–832.
- Luzardo L, Lujambio I, Sottolano M, et al. 24-h ambulatory recording of aortic pulse wave velocity and central systolic augmentation: a feasibility study. Hypertens Res. 2012;35:980–987.
- Weiss W, Gohlisch C, Harsch-Gladisch C, et al. Oscillometric estimation of central blood pressure: validation of the Mobil-O-Graph in comparison with the SphygmoCor device. Blood Press Monit. 2012;17:128–131.
- Hametner B, Wassertheurer S, Kropf J, et al. Oscillometric estimation of aortic pulse wave velocity: comparison with intra-aortic catheter measurements. Blood Press Monit. 2013;18:173–176.
- Benas D, Kornelakis M, Triantafyllidi H, et al. Pulse wave analysis using the Mobil-O-Graph, Arteriograph and Complior device: a comparative study. Blood Press. 2019;28:107–113.
- Protogerou AD, Antonis A, Efthymia N, et al. Feasibility and reproducibility of noninvasive 24-h ambulatory aortic blood pressure monitoring with a brachial cuff-based oscillometric device. Am J Hypertens. 2012;25:876–882.
- Papaioannou TG, Argyris A, Protogerou AD, et al. Non-invasive 24 hour ambulatory monitoring of aortic wave reflection and arterial stiffness by a novel oscillometric device: the first feasibility and reproducibility study. Int J Cardiol. 2013;169:57–61.
- Eeftinck Schattenkerk DW, van Gorp J, Snijder MB, et al. Ethnic differences in arterial wave reflection are mostly explained by differences in body height - cross-sectional analysis of the HELIUS Study. PLoS One. 2016;11:e0160243.
- Sheng CS, Cheng YB, Wei FF, et al. Diurnal blood pressure rhythmicity in relation to environmental and genetic cues in untreated referred patients. Hypertension. 2017;69:128–135.
- Parati G, Stergiou G, O'Brien E, et al. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens. 2014;32:1359–1366.
- Li Y, Wang JG, Gao PJ, et al. Interaction between body mass index and alcohol intake in relation to blood pressure in HAN and SHE Chinese. Am J Hypertens. 2006;19:448–453.
- Inker LA, Schmid CH, Tighiouart H, et al. CKD-EPI Investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29.
- Williams B, Mancia G, Spiering W, et al. 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. Blood Press. 2018;27:314–340.
- Krzesiński P, Stańczyk A, Gielerak G, et al. Sex determines cardiovascular hemodynamics in hypertension. J Hum Hypertens. 2015;29:610–617.
- Stergiou GS, Kollias A, Rarra VC, et al. Ambulatory arterial stiffness index: reproducibility of different definitions. Am J Hypertens. 2010;23:129–134.
- Dechering DG, van der Steen MS, Adiyaman A, et al. Reproducibility of the ambulatory arterial stiffness index in hypertensive patients. J Hypertens. 2008;26:1993–2000.
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327:307–310.
- Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for prevention, detection, evaluation, and management of high blood pressure in Adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;71:e127–e248.
- Stenehjem AE, Os I. Reproducibility of blood pressure variability, white-coat effect and dipping pattern in untreated, uncomplicated and newly diagnosed essential hypertension. Blood Press. 2004;13:214–224.
- Wizner B, Dechering DG, Thijs L, et al. Short-term and long-term repeatability of the morning blood pressure in older patients with isolated systolic hypertension. J Hypertens. 2008;26:1328–1335.
- Eguchi K, Hoshide S, Hoshide Y, et al. Reproducibility of ambulatory blood pressure in treated and untreated hypertensive patients. J Hypertens. 2010;28:918–924.
- Cuspidi C, Meani S, Valerio C, et al. Reproducibility of dipping/nondipping pattern in untreated essential hypertensive patients: impact of sex and age. Blood Press Monit. 2007;12:101–106.
- Palatini P, Mormino P, Canali C, et al. Factors affecting ambulatory blood pressure reproducibility. Results of the HARVEST Trial. Hypertension and Ambulatory Recording Venetia Study. Hypertension. 1994;23:211–216.
- Gerin W, Rosofsky M, Pieper C, et al. A test of reproducibility of blood pressure and heart rate variability using a controlled ambulatory procedure. J Hypertens. 1993;11:1127–1131.
- de la Sierra A, Vinyoles E, Banegas JR, et al. Short-term and long-term reproducibility of hypertension phenotypes obtained by office and ambulatory blood pressure measurements. J Clin Hypertens. 2016;18:927–933.
- Narkiewicz K, Kjeldsen SE, Burnier M, et al. Challenges in oscillometric blood pressure measurement in atrial fibrillation: looking for practical solutions. Blood Press. 2018;27:1–2.
- Ohta Y, Kawano Y, Hayashi S, et al. Effects of cigarette smoking on ambulatory blood pressure, heart rate, and heart rate variability in treated hypertensive patients. Clin Exp Hypertens. 2016;38:510–513.
- Agabiti-Rosei E, Mancia G, O'Rourke MF, et al. Central blood pressure measurements and antihypertensive therapy: a consensus document. Hypertension. 2007;50:154–160.
