Abstract
Purpose: Non-dipping blood pressure (BP) pattern has been associated with metabolic changes and cardiovascular events. With regard of diabetes, studies are scarce. Our aim was to investigate if there is an association between changes in dipping patterns and incidence of diabetes.
Materials and methods: A 24-h ambulatory BP measurement was recorded in addition to other laboratory measurements, and a questionnaire and physical examination were carried out in the baseline study and after 21-year follow-up among a study population (n = 449) consisting of randomly selected middle-aged Finnish females and males without diabetes.
Results: 128 (28.5%) developed diabetes during the follow-up. The incidence of new-onset diabetes was the highest, 41.0%, among those subjects who were non-dippers (their systolic BP declined <10% from daytime to nighttime) in the baseline and also in the follow-up study, while the incidence of diabetes was 19.6% in the dipper – dipper (a nighttime decline of systolic BP 10% or more) group (p = 0.003). The difference remained statistically significant after adjustment with age, sex, body mass index, fasting glucose, triglycerides, and insulin levels, smoking status, 24-h mean systolic BP, high-sensitivity C-reactive protein, estimated glomerular filtration and diuretics use. In logistic regression analysis, the non-dipper – non-dippers were at higher risk of diabetes compared with dipper – dipper group (OR = 2.27, 95% CI: 1.13–4.56, p = 0.022).
Conclusions: Our prospective study shows that there is an independent association between non-dipping BP pattern and the incidence of diabetes in a 21-year follow-up.
Introduction
Ambulatory blood pressure measurement (ABPM) is a method consisting of repeated blood pressure (BP) measurements during daytime and nighttime, providing an assessment of mean BP and diurnal pattern of BP variation over 24 h. Physiologically most individuals have a clear circadian BP rhythm characterized by a nighttime drop of 10–20% in both systolic and diastolic BP level. BP dropping by night is called dipping. Among some subjects the nocturnal decline is lacking. This phenomenon is called non-dipping [Citation1]. Non-dipping pattern has been associated with several cardiovascular risk factors [Citation2], metabolic syndrome [Citation3], and other target organ damage such as chronic kidney disease [Citation4]. Ambulatory mean systolic BP has been shown to predict cardiovascular events better than clinic BP in previous population studies [Citation5], especially nighttime systolic BP [Citation6]. In Dublin Outcome Study with a cohort of over 11,000 diabetic patients with a mean follow-up of 5.3 years, nighttime systolic BP was a significant predictor of cardiovascular (CV) mortality [Citation7]. In another study with diabetic patients, non-dipping was recognized as an independent risk factor for cardiovascular events and mortality even in normotensive subjects [Citation8]. In a recent large register-based study with a 5.7-year of follow-up, ambulatory systolic BP was a stronger predictor of all-cause and cardiovascular mortality than clinic systolic BP [Citation9].
In most follow-up studies with ABPM, the target of interest has been all-cause and CV mortality or CV events. Median follow-up times have been up to 10 years [Citation10]. Lately, there have also appeared larger studies with longer follow-up times [Citation11]. As far as we know, follow-up studies with dipping status as a predictor of new-onset diabetes are scarce. Hermida et al. reported in their MAPEC study that abnormal sleep-time BP determined by ABPM was independently associated with new-onset diabetes during a 5.6-year follow-up [Citation12]. They also carried out a randomized controlled trial with the finding of blood pressure medication ingested in bed-time resulting in reduction of new-onset type 2 diabetes [Citation13]. ABPM is recommended to identify varying BP-profiles. There has been increasing interest especially in nocturnal BP values, and a new recommendation for ABPM use has been published [Citation14].
In this present study, we investigated changes in dipping status and the association of dipping status with new-onset diabetes during a 21-year follow-up in a randomly selected population-based cohort (n = 449) of middle-aged Finnish subjects.
Materials and methods
This study is part of the OPERA (Oulu Project Elucidating Risk of Atherosclerosis) project. The details have been published earlier [Citation15]. In short, a cohort of 600 hypertensive 40–59 years old females and males were randomly selected from the population of city of Oulu in Northern Finland. The control cohort consisting of 600 subjects was matched for age and sex. Altogether, 1045 individuals (87%) participated in the baseline study which was carried out between 1990 and 1993. In the second phase, OPERA has been converted to a prospective study by convening the participants in a follow-up visit between 2013 and 2014. The mean follow-up time was 21.7 years (interquartile range 1.1). Of the 813 survivors, a total of 600 subjects attended, 310 from the control cohort and 290 from the hypertensive cohort. In the present study, we included only subjects that had ABPM recorded both in the baseline study and in the follow-up study. Individuals with diabetes were selected out. Thus, the study population of this investigation consisted of 449 subjects (228 female, 221 male).
The same examinations were performed at the baseline and in the follow-up survey, including anthropometric measurements, blood tests and questionnaires. The examinations have been described in more detail previously [Citation3,Citation16]. The estimated glomerular filtration rate (eGFR) was calculated by using CKD-EPI equation [Citation17]. Type 2 diabetes was determined by the WHO criteria [Citation18]: diabetes was diagnosed if fasting plasma glucose was ≥7.0 mmol/L and/or 2-h plasma glucose was ≥11.1 mmol/L in 2-h oral glucose tolerance test, or if a person was on medication for diabetes.
Hypertension was defined as blood pressure over 140/90 mmHg or current use of antihypertensive medication.
The presence of the metabolic syndrome was defined according to the International Diabetes Federation (IDF) definition [Citation19]. Main criteria is the central obesity (waist circumference ≥94 cm for male, ≥80 cm for female of European origin), in addition to any two of the following factors: raised triglycerides levels (≥1.7 mmol/L) or specific treatment for this lipid abnormality; reduced HDL cholesterol levels (<1.03 mmol/L in males, <1.29 mmol/L in females) or specific treatment for this lipid abnormality; elevated blood pressure (systolic BP ≥130 or diastolic BP ≥85 mmHg) or treatment of previously diagnosed hypertension; raised fasting plasma glucose (≥5.6 mmol/L) or previously diagnosed type 2 diabetes (subjects with diabetes were excluded in the present study).
The office BP was measured using an automatic oscillometric device (Dinamap Procare 100, Criticon, Tampa, Florida, USA) [Citation20] when the subjects were seated for the minimum of 5 min. BP was then measured at 1 min intervals three times. The mean of the second and the third measurement was used for the analysis.
A noninvasive fully automatic SpaceLabs90207 oscillometric unit (SpaceLabs Inc., Redmond, Washington, USA) was used to record ABPM in the baseline study. The device was set to take a measurement every 15 min between 04:00 AM to 12:00 PM and every 20 min between 12:00 PM to 04:00 AM. The British Hypertension Society and the US Association for the Advancement of Medical Instrumentation have previously confirmed the accuracy and reproducibility of the BP readings acquired with this device. The proper positioning of the cuff was ensured in each individual by means of the similarity (difference <5 mmHg) between four SpaceLabs BP measurements and four auscultatory readings using a Y-connector and the patients were instructed to relax their arm during the measurement. Values were automatically excluded from the analysis if systolic BP (SBP) was less than 70 or more than 250 mmHg, diastolic BP (DBP) less than 40 or more than 150 mmHg, and heart rate less than 40 or more than 150 bpm. Less than 3% of the BP readings were rejected as artifacts on the basis of these criteria. During the follow-up period the ABPM was recorded with Oscar 2 oscillometric ambulatory BP monitor (SunTech Medical, Morrisville, North Carolina, USA) [Citation21] with the same protocol as in the baseline study. AccuWin Pro™ V3 software was used in the analysis of ABPM data. A decline of systolic BP from daytime to nighttime <10% was considered as non-dipping pattern of BP.
This study was approved by the Ethics Committee of the Faculty of Medicine, University of Oulu. All work has been conducted in accordance with the Declaration of Helsinki. All study subjects gave informed consent.
Statistical methods
Data analyses were performed with IBM SPSS Statistics for Windows (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY, USA: IBM Corp.) [Citation22]. Baseline data are expressed as prevalence, mean ± SD for continuous variables, or median with 25th and 75th quartiles for skewed variables. Continuous variables were tested for difference between the groups with Student’s t-test or with Mann-Whitney’s test. For categorical variables Pearson’s Chi-square test was used. Log transformation was made for skewed variables. Differences between different dipping groups were tested by using ANOVA Tukey test (post hoc analysis). Binary logistic regression was used to assess the association of dipping status with the incidence of new-onset diabetes.
Results
During the follow-up time, 128 (28.5%) participants developed diabetes. Of them, 46.9% (n = 60) were women.
The characteristics of the study population at baseline are presented in divided by whether diabetes was developed during the follow-up time or not. Those who developed diabetes had more often unfavourable metabolic characteristics and life-style factors compared with those who remained non-diabetic: 47.7% of those who developed diabetes met the IDF criteria for metabolic syndrome in the baseline study, whereas only 19.6% of those who remained non-diabetic (p < 0.001). Of the study population, 46% was on antihypertensive medication. It was more frequent in those, who developed diabetes compared with those who remained non-diabetics (p = 0.001), and the difference was statistically significant for ACE (angiotensin-converting enzyme) inhibitors (p = 0.002), calcium channel blockers (p = 0.029) and diuretics (p = 0.003) but not for beta blockers. The majority (61.4%) used only one antihypertensive drug, 30.9% used two drugs and only 7.7% had three drugs. There were more new diabetics among those who used two or more drugs (p = 0.012).
Table 1. Baseline characteristics and new-onset diabetes during 21-year follow-up.
In the baseline study, 22.7% (n = 102) of the cohort were non-dippers. In the follow-up study, subjects were further divided by the dipping status into four groups as follows: Group 1 consisted of those, who had dipping of the systolic blood pressure in both the baseline and follow-up study (dipper – dipper; n = 148, 33.0%). Group 2 consisted of those whose dipping status changed from non-dipping to dipping (non-dipper – dipper; n = 19, 4.2%). Those who showed dipping at baseline, but did not have it in the follow up (dipper – non-dipper; n = 199, 44.3%) fell into group 3. If there was no dipping during the baseline nor the follow-up study, subjects formed group 4 (non-dipper – non-dipper; n = 83, 18.5%). Of all dippers in the cross-sectional study, 42.7% remained dippers, while of all non-dippers, 81.4% remained non-dippers in the follow-up.
The main characteristics of each group are presented in . Non-dipper – non-dippers were older (p = 0.006), were more frequently hypertensive (p < 0.001), had more frequently antihypertensive medication (p < 0.001), had greater body mass index (p = 0.003), had higher systolic (p = 0.009) and diastolic blood pressure (p = 0.002) than dipper – dippers. Also total fasting cholesterol (p = 0.001) and triglyceride levels (p = 0.018) were higher in non-dipper – non-dippers compared with dipper – dippers. Estimated glomerular filtration was higher (p = 0.017) and creatinine was lower (p = 0.03) in dipper – dipper group than in non-dipper – non-dipper group, although there were no differences in baseline creatinine levels nor eGFR in those who developed diabetes compared with those who did not (). In our study population, only 5% had decreased glomerular filtration rate (<60 mL/min). In post hoc analyses, there were statistical differences also between dipper – non-dipper and non-dipper – non-dipper groups: the latter were more often hypertensive using antihypertensive medication more frequently, their BMI and triglycerides levels were higher, and their eGFR was lower (). There was a statistically significant difference in ACE inhibitor use (p = 0.023) and in calcium channel blocker use (p = 0.003) in the non-dipping – non-dipping group compared with the other groups, but not in diuretics or beta blocker use.
Table 2. Baseline characteristics according to dipping status.
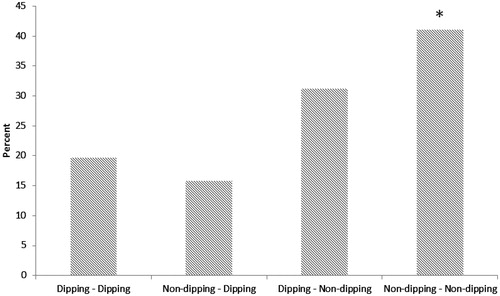
The incidence of new diabetes cases were 19.6% (29/148) in dipper – dipper group, 15.8% (3/19) in non-dipper – dipper group, 31.2% (62/199) in dipper – non-dipper group and finally, 41.0% (34/83) in non-dipper – non-dipper group (p = 0.003), respectively ().
Figure 1. Incidence of diabetes (%) according to dipping status. *p < 0.001 is for the Mantel-Haenszel linear-by-linear association.
The association between the dipping status and the development of new diabetes was assessed also by using binary logistic regression analysis. The effect of confounding factors significant in univariate analyses were controlled for by adding them into the multivariate model. The following variables were entered into the multivariate model as covariates: age, sex, body mass index, fasting glucose levels, fasting insulin levels, fasting triglycerides levels, smoking status and 24-h mean systolic BP (). Non-dipper – non-dipper status was a statistically significant predictor of new diabetes in logistic regression analysis so that the subjects belonging to that group were at higher risk for getting new diabetes during the follow-up time (OR (odds ratio) = 2.27, 95% CI: 1.13–4.55, p = 0.022) compared to the subjects who remained dippers. The statistically significant difference remained after adjustment for metabolic syndrome and 24-h ambulatory mean systolic BP. Also dipping – non-dipping group had increased risk for new diabetes (OR =1.90, 95% CI: 1.06–3.38, p = 0.03) compared with the constant dipper group. Adding hsCRP (high-sensitivity C-reactive protein), creatinine, eGFR or diuretics use to model did not change these results significantly.
Table 3. Binary logistic regression model on the new-onset diabetes in 21-year follow-up.
In addition to non-dipping status, baseline increased fasting glucose levels (≥5.6 mmol/l) was strongly associated with the risk for new diabetes (OR = 9.92, 95% CI: 4.23 – 23.29, p < 0.001). Also high (≥1.7 mmol/L) fasting triglycerides levels (OR = 1.77, 95% CI: 1.03–3.06, p = 0.04) or higher fasting insulin levels (OR = 1.04, 95% CI: 1.00–1.08, p = 0.03) indicated enhanced risk for diabetes.
During the follow-up, some of the metabolic variables as well as blood pressure of the non-dipper – non-dipper group changed into more favourable levels (). Systolic and diastolic blood pressure (p < 0.001), total fasting cholesterol (p = 0.002), LDL cholesterol (p = 0.039) and triglycerides levels (p = 0.008) decreased more among non-dipper – non-dippers than dipper – dippers. They also had more often antihypertensive and lipid medication (p < 0.001).
Table 4. Change in variables during the 20-year follow-up and dipping status.
Discussion
Major novel finding of the present study suggests that non-dipping – non-dipping BP pattern in ABPM is associated with the incidence of diabetes during the 21-year follow-up in a randomly selected Finnish, originally middle-aged population. Different ambulatory BP patterns have rarely been investigated as predictors of diabetes. In PAMELA study (Pressioni Arteriose Monitorate E Loro Associazioni), 1412 non-diabetic individuals were classified according to ABPM as normotensives, sustained hypertensive, white coat hypertensives (WCH) or masked hypertensives (MHT). They were followed-up over 10-year period. The incidence of diabetes was substantially greater in subjects with WCH, MHT and sustained hypertension than in the normotensive group [Citation23]. Dipping status was not, however, considered in this study.
In a Chinese cross-sectional study, 531 hypertensive subjects were evaluated with ABPM, and 25% of them were diagnosed as diabetics. It was found out that reverse dippers, a variant of non-dipping pattern with higher mean nighttime BP than daytime BP, and non-dippers had higher prevalence of type 2 diabetes than dippers [Citation24]. We have previously shown in a cross-sectional study design of the OPERA cohort that non-dipping BP pattern is associated with metabolic abnormalities [Citation3] and in renal function [Citation25]. In the present study, there were significant differences in several metabolic factors already in cross-sectional study between those who developed diabetes compared with those who remained non-diabetic: They were more often overweight and their office blood pressure as well as ambulatory 24-h mean systolic BP were higher although they had more frequently antihypertensive medication. Although new diabetics were on ACE inhibitor or diuretic medication more frequently compared with the non-diabetics, medications did not have an influence on the development of diabetes in the multivariate model. There were also differences in antihypertensive medication use according to dipping status: non-dippers had more often ACE inhibitor or diuretic medication compared with others, but neither medication were independently associated with the development of diabetes. The diuretic in use was mainly hydrochlorothiazide, only under 1% of the study population used loop diuretic. In a short follow-up study with ABPM [Citation26], non-dippers were observed to respond well to both hydrochlorothiazide and lisinopril, while dippers responded to lisinopril, but not to hydrochlorothiazide. Among the future diabetics, also fasting glucose, triglycerides and insulin levels were higher and HDL cholesterol levels lower. Thus the metabolic profile was unfavorable in those who developed diabetes; almost half of them met the IDF criteria for metabolic syndrome [Citation19]. However, non-dipping status remained as a prognostic factor for diabetes even after adjustment for metabolic syndrome and 24-h mean systolic BP. Our findings are in accordance with an earlier population-based study in middle-aged patients with hypertension in which the prevalence of non-dipping pattern was significantly greater in patients belonging to high cardiovascular risk category [Citation2]. In a cross-sectional study with subjects with uncontrolled hypertension identified by 48-h ABPM [Citation27], a potential impact of the time of the day of antihypertensive medication on the dipping status and metabolic factors was observed: among those subjects who were taking one antihypertensive drug at bedtime compared with patients receiving all drugs on awakening, the prevalence of non-dipping was lower. They also had lower blood glucose, cholesterol and fibrinogen levels and lower urinary albumin excretion. In addition to lowering the cardiovascular risk, episodes of harmful hypotension could be avoided by taking at least one of the antihypertensive medications at bedtime [Citation28]. Unfortunately, in our study, there is no data on timing of antihypertensive medication.
It has been previously shown in chronic kidney disease patients that they present twice as often reverse dipping (e.g. nighttime peak) than subjects with normal kidney function [Citation29]. Alterations in kidney function have been associated with endogenous inhibitors of nitric oxide such as ADMA (asymmetric dimethylarginine). Reduced bioavailability of nitric oxide is a potential mechanism for salt sensitivity of BP and may cause an abnormal pattern of vascular reactivity [Citation30]. Although the eGFR was lower in the non-dipping – non-dipping group, there was no association of eGFR with the development of diabetes in multivariate analysis in our study. There were also no statistically significant differences in the baseline creatinine and eGFR between those who developed diabetes or who did not.
Gender difference in the blood pressure variability profile has been reported [Citation31]. In our study, there were gender differences in blood pressure levels: men had higher blood pressures in office measurements as well as in 24-h ABPM, as expected. All the analyses were adjusted by sex, although it was not associated with the development of diabetes.
How could non-dipping phenomenon increase the risk for metabolic abnormalities? Early endothelial dysfunction and its influence on vascular reactivity have been detected in normoglycemic first-degree relatives of diabetic patients independent of the metabolic syndrome [Citation32]. Non-dipping pattern of BP possibly also related to vascular reactivity could thus be another facet of the metabolic alterations. Another condition linking non-dipping pattern and vascular reactivity could be chronic, low-grade inflammation. The latter notion is supported by earlier data [Citation33] founding an association between metabolic syndrome and circulating cytokine levels with accelerated arterial thickening and stiffening. However, although hsCRP was increased in subjects who developed diabetes, it was not an independent predictor in multivariate model in the present study.
Rahman et al. [Citation34] address in their comprehensive review that the circadian rhythm is maintained by the circadian timing system with cycling molecular components in hypothalamus and also in peripheral target organs (heart, kidneys, vasculature). In animal models and also in humans, alterations in the central clock activity are associated with metabolic abnormalities [Citation35], impaired function of autonomic nervous system, RAS system and organ malfunction in target organs [Citation36]. These changes play a significant role in development of non-dipping BP pattern and associated CV events [Citation34].
In the present study, a categorized variable for the nighttime dipping was used, as has been done in the majority of previous literature [Citation10]. Definition of dipping as a categorical compared with continuous variable has been criticized as an arbitrary divide of the variable [Citation10]. However, for our study it was more suitable to use a categorical variable since we were interested in the changes in the dipping status during long-term follow-up.
There has been debate over reproducibility of BP dipping in ABPM. In a study by Hinderliter et al., 24-h BP monitoring was performed in three occasions in 115 untreated adult subjects. They concluded that there was substantial day-to-day variability in BP dipping although mean 24-h and awake BPs were fairly stable. Differences in day-to-day dipping were considered present due to sleep quality [Citation37]. In this study, we gathered no information of the quality of sleep, nor the prevalence of sleep apnea. In another study, 65 untreated newly-diagnosed hypertensive subjects underwent ABPM twice with 4-week interval, and the nocturnal dipping pattern was found unchanged in 82% of the patients [Citation38]. In a retrospective ABPM database study with over 500 untreated patients, two consecutive ABPM recordings were obtained approximately 2.5 years apart. Dipper/non-dipper status was more reproducible when using dipping as a continuous variable than as a dichotomous variable, yet dipper/non-dipper status was unchanged in 76% of patients [Citation39]. The latter is in accordance with previous studies with shorter time frame and fewer subjects [Citation40–42]. In the present study, non-dipper status was more stable than dipper status. The consistence of dipping status in consecutive days has been also lower in some studies [Citation43]. Cuspidi et al. investigated the intra subject variability of a non-dipping pattern in a group of type 2 diabetic hypertensives and untreated non-diabetic hypertensive subjects [Citation2]. Non-dipping pattern was highly reproducible in diabetic patients. The prevalence of impaired circadian BP pattern was approximately three-fold higher in diabetic hypertensive group than in non-diabetic group. They found that classification of hypertensive patients as dippers or non-dippers is more reliable in diabetics than in non-diabetics even in a single ABP recording. Furthermore, they concluded that cardiac and extra cardiac target organ damage was associated with more frequent and reproducible non-dipping pattern in diabetic patients.
It has been postulated that pulse pressure reduction from the daytime to the nighttime might have explanatory value in diurnal BP pattern [Citation44]. In our study, there were no significant differences in pulse pressure reduction from the daytime to the nighttime between those who developed diabetes compared with those who remained non-diabetic.
Our study has some limitations. First, fixed-clock intervals were used in defining the daytime and the nighttime and the transitional times in the morning and in the evening were not excluded in the analyses. Satoh et al compared ABPM fixed-clock intervals (daytime 09:00–21:00 and nighttime 01:00–06.00) with diary records to identify nocturnal BP dipping pattern as a predictor of CV disease risk in a 17-year follow-up. In this Japanese study they found out that non-dippers, extreme dippers (BP dipping over 20%), and risers were in greater CV disease risk than dippers. They also concluded that while diary records are preferable, the standard fixed-time intervals also are suitable in population-based studies [Citation11]. Second, two different blood pressure measurement equipment were used during the baseline study and the follow-up examination. There is no data of intervariability of these devices, but both the appliances were previously validated according to the internationally accepted validation protocols. Third, the study population consisted of Caucasian subjects only, which is clearly a limitation, but nonetheless represents the Finnish homogenous population. Last, information on the time of onset of diabetes is not available.
In conclusion, this population-based prospective study on middle-aged individuals suggests that non-dipping pattern of nighttime BP is associated with incidence of new-onset diabetes in over 20 years of follow-up. Home or clinic BP measurements cannot provide the information of nighttime BP, therefore, the ABPM could be a valuable additional tool for identifying patients in future risk for diabetes. Those with non-dipping pattern of BP could benefit from regular screening of glucose metabolism for early detection of diabetes.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- O’Brien E, Sheridan J, O’Malley K. Dippers and non-dippers. Lancet. 1988;2:397.
- Cuspidi C, Meani S, Valerio C, et al. Nocturnal non-dipping pattern in untreated hypertensives at different cardiovascular risk according to the 2003 ESH/ESC guidelines. Blood Press. 2006;15:37–44.
- Ukkola O, Vasunta R, Kesäniemi YA. Non-dipping pattern in ambulatory blood pressure monitoring is associated with metabolic abnormalities in a random sample of middle-aged subjects. Hypertens Res. 2009;32:1022–1027.
- Minutolo R, Agarwal R, Borrelli S, et al. Prognostic role of ambulatory blood pressure measurement in patients with nondialysis chronic kidney disease. Arch Intern Med. 2011;171:1090–1098.
- Hansen TW, Jeppesen J, Rasmussen S, et al. Ambulatory blood pressure and mortality: a population-based study. Hypertension. 2005;45:499–504.
- Kikuya M, Ohkubo T, Asayama K, et al. Ambulatory blood pressure and 10-year risk of cardiovascular and noncardiovascular mortality: the Ohasama study. Hypertension. 2005;45:240–245.
- Draman MS, Dolan E, van der Poel L, et al. The importance of night-time systolic blood pressure in diabetic patients: Dublin Outcome Study. J Hypertens.2015;33:1373–1377.
- Ohkubo T, Hozawa A, Yamaguchi J, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens.2002;20:2183–2189.
- Banegas JR, Ruilope LM, de la Sierra A, et al. Relationship between clinic and ambulatory blood-pressure measurements and mortality. N Engl J Med. 2018;378:1509–1520.
- Taylor KS, Heneghan CJ, Stevens RJ, et al. Heterogeneity of prognostic studies of 24-hour blood pressure variability: systematic review and meta-analysis. PLoS One. 2015;10:e0126375.
- Satoh M, Asayama K, Kikuya M, et al. Nocturnal blood pressure decline based on different time intervals and long-term cardiovascular risk: the Ohasama Study. Clin Exp Hypertens. 2018;40:1–7.
- Hermida RC, Ayala DE, Mojón A, et al. Sleep-time BP: prognostic marker of type 2 diabetes and therapeutic target for prevention. Diabetologia 2016;59:244–254.
- Hermida RC, Ayala DE, Mojón A, et al. Bedtime ingestion of hypertension medications reduces the risk of new-onset type 2 diabetes: a randomised controlled trial. Diabetologia 2016;59:255–265.
- Parati G, Stergiou G, O’Brien E, et al. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens. 2014;32:1359–1366.
- Rantala AO, Kauma H, Lilja M, et al. Prevalence of the metabolic syndrome in drug-treated hypertensive patients and control subjects. J Intern Med. 1999;245:163–174.
- Leinonen T, Kesäniemi YA, Hedberg P, et al. Serum ghrelin and prediction of metabolic parameters in over 20-year follow-up. Peptides 2016;76:51–56.
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612.
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553.
- Alberti KG, Zimmet P, Shaw J. Metabolic syndrome–a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006. 23:469–480.
- Reinders A, Reggiori F, Shennan AH. Validation of the DINAMAP ProCare blood pressure device according to the international protocol in an adult population. Blood Press Monit. 2006;11:293–296.
- Goodwin J, Bilous M, Winship S, et al. Validation of the Oscar 2 oscillometric 24-h ambulatory blood pressure monitor according to the British Hypertension Society protocol. Blood Press Monit. 2007;12:113–117.
- IBM Corp. IBM SPSS Statistics for Windows, Version 25.0. 2017.
- Mancia G, Bombelli M, Facchetti R, et al. Increased long-term risk of new-onset diabetes mellitus in white-coat and masked hypertension. J Hypertens. 2009;27:1672–1678.
- Sun L, Yan B, Gao Y, et al. Relationship between blood pressure reverse dipping and type 2 diabetes in hypertensive patients. Sci Rep. 2016;6:25053.
- Kastarinen H, Vasunta RL, Ukkola O, et al. Glomerular filtration rate is related to dipping pattern in ambulatory blood pressure monitoring–a cross-sectional population-based study. J Hum Hypertens. 2010;24:247–253.
- Weir MR, Reisin E, Falkner B, et al. Nocturnal reduction of blood pressure and the antihypertensive response to a diuretic or angiotensin converting enzyme inhibitor in obese hypertensive patients. TROPHY Study Group. Am J Hypertens. 1998;11:914–920.
- Hermida RC, Ayala DE, Calvo C, et al. Effects of time of day of treatment on ambulatory blood pressure pattern of patients with resistant hypertension. Hypertension. 2005;46:1053–1059.
- Scuteri A, Tesauro M, Guglini L, et al. Aortic stiffness and hypotension episodes are associated with impaired cognitive function in older subjects with subjective complaints of memory loss. Int J Cardiol. 2013;169:371–377.
- Di Daniele N, Fegatelli DA, Rovella V, et al. Circadian blood pressure patterns and blood pressure control in patients with chronic kidney disease. Atherosclerosis 2017;267:139–145.
- Scuteri A, Stuehlinger MC, Cooke JP, et al. Nitric oxide inhibition as a mechanism for blood pressure increase during salt loading in normotensive postmenopausal women. J Hypertens. 2003;21:1339–1346.
- Scuteri A, Morrell CH, Orru’ M, et al. Gender specific profiles of white coat and masked hypertension impacts on arterial structure and function in the SardiNIA study. Int J Cardiol. 2016;217:92–98.
- Scuteri A, Tesauro M, Rizza S, et al. Endothelial function and arterial stiffness in normotensive normoglycemic first-degree relatives of diabetic patients are independent of the metabolic syndrome. Nutr Metab Cardiovasc Dis. 2008;18:349–356.
- Scuteri A, Orru M, Morrell C, et al. Independent and additive effects of cytokine patterns and the metabolic syndrome on arterial aging in the SardiNIA Study. Atherosclerosis 2011;215:459–464.
- Rahman A, Hasan AU, Nishiyama A, et al. Altered circadian timing system-mediated non-dipping pattern of blood pressure and associated cardiovascular disorders in metabolic and kidney diseases. IJMS. 2018;19:400.
- Sookoian S, Gianotti TF, Burgueno A, et al. Gene-gene interaction between serotonin transporter (SLC6A4) and CLOCK modulates the risk of metabolic syndrome in rotating shiftworkers. Chronobiol Int. 2010;27:1202–1218.
- Young ME, Wilson CR, Razeghi P, et al. Alterations of the circadian clock in the heart by streptozotocin-induced diabetes. J Mol Cell Cardiol. 2002;34:223–231.
- Hinderliter AL, Routledge FS, Blumenthal JA, et al. Reproducibility of blood pressure dipping: relation to day-to-day variability in sleep quality. J Am Soc Hypertens. 2013;7:432–439.
- Stenehjem AE, Os I. Reproducibility of blood pressure variability, white-coat effect and dipping pattern in untreated, uncomplicated and newly diagnosed essential hypertension. Blood Press. 2004;13:214–224.
- McGowan NJ, Gough K, Padfield PL. Nocturnal dipping is reproducible in the long term. Blood Press Monit. 2009;14:185–189.
- Mochizuki Y, Okutani M, Donfeng Y, et al. Limited reproducibility of circadian variation in blood pressure dippers and nondippers. Am J Hypertens. 1998;11:403–409.
- Keren S, Leibowitz A, Grossman E, et al. Limited reproducibility of 24-h ambulatory blood pressure monitoring. Clin Exp Hypertens. 2015;37:599–603.
- Cuspidi C, Meani S, Lonati L, et al. Short-term reproducibility of a non-dipping pattern in type 2 diabetic hypertensive patients. J Hypertens. 2006;24:647–653.
- Omboni S, Parati G, Palatini P, et al. Reproducibility and clinical value of nocturnal hypotension: prospective evidence from the SAMPLE study. Study on Ambulatory Monitoring of Pressure and Lisinopril Evaluation. J Hypertens. 1998;16:733–738.
- Mancia G, Verdecchia P. Clinical value of ambulatory blood pressure: evidence and limits. Circ Res. 2015;116:1034–1045.
