Abstract
Aims: Atrial fibrillation (AF) is associated with increased cardiovascular risk and the incidence increases with age, hypertension and left ventricular hypertrophy (LVH). Reducing in-treatment systolic blood pressure (SBP) prevents new-onset AF but has previously not been studied in patients with isolated systolic hypertension (ISH). We aimed to investigate the effect on preventing new-onset AF by decreased in-treatment SBP in patients with ISH compared to patients with non-ISH.
Methods and results: Double-blind, randomized, parallel-group study of 1320 patients with ISH and electrocardiographic (ECG) LVH, included among the 9193 patients in the Losartan Intervention For Endpoint reduction in hypertension (LIFE) study. Annual ECGs were Minnesota coded centrally, and new-onset AF was evaluated in 1248 ISH patients and compared with 7583 non-ISH patients during mean 4.8 ± 0.9 years follow-up. Cox regression analyses were used to assess the effect of reduced in-treatment SBP. New-onset AF occurred in 61 (4.9%) ISH patients and 292 (3.9%) non-ISH patients. In multivariate analysis lower in-treatment SBP was associated with 17% risk reduction (p = 0.008) for new-onset AF in ISH patients and 9% risk reduction (p = 0.006) in non-ISH patients per 10 mmHg decrease in in-treatment SBP, independent of treatment modality, baseline risk factors, baseline SBP and in-treatment heart rate and ECG-LVH. There was a significant interaction (p = 0.041) in favor of SBP reduction and AF prevention in ISH vs. non-ISH patients.
Conclusion: Our data suggest that the effect of in-treatment SBP reduction in preventing new-onset AF is stronger in ISH compared to non-ISH patients with hypertension and ECG-LVH. However, the principal findings were the same in ISH and non-ISH patients.
Introduction
Isolated systolic hypertension (ISH), usually the most common form of hypertension in the elderly, is associated with increased risk of cardiovascular morbidity and mortality compared to systolic-diastolic and isolated diastolic (non-ISH) hypertension [Citation1,Citation2], and antihypertensive treatment is efficacious in preventing stroke and myocardial infarction in ISH patients [Citation3].
The Losartan Intervention For Endpoint reduction in hypertension (LIFE) study comprised of 9193 patients aged 55–80 years., with hypertension and electrocardiographic left ventricular hypertrophy (ECG-LVH), followed for 4.8 ± 0.9 years. who were randomized to losartan vs. atenolol targeting systolic blood pressure (SBP) <140 mmHg [Citation4]. Losartan reduced new-onset atrial fibrillation (AF), a secondary endpoint and subsequent stroke compared to atenolol [Citation5] and lower Cornell product and lower heart rate over time were associated with less new-onset AF [Citation6,Citation7].
Patients with ISH comprised a pre-specified subgroup of special interest in the LIFE Study [Citation8]. We have shown that the LIFE patients with ISH benefitted from regression of LVH in response to the antihypertensive treatment [Citation9,Citation10] and that pulse pressure at baseline and during treatment were predictors of incident AF [Citation11]. Whether lower in-treatment SBP is associated with less AF in patients with ISH and ECG-LVH has, however, not been specifically investigated.
Based on the benefit of LVH regression in preventing incident AF [Citation7], the benefit in the ISH patients of regression of LVH on endpoint protection [Citation9,Citation10] and the association between lower pulse pressure during treatment and incident AF [Citation11], we hypothesized and investigated whether patients with ISH would benefit from the antihypertensive treatment in preventing AF compared to non-ISH patients who participated in the LIFE study. Competing risk was additionally analyzed with incident AF and mortality as a combined endpoint.
Material and methods
Participants and study design
As described in detail elsewhere [Citation1,Citation12–14], the LIFE study enrolled patients with hypertension having ECG-LVH determined by Cornell voltage-duration product [Citation15–17] and/or Sokolow-Lyon voltage criteria [Citation18] on a screening ECG in a prospective, double-blind, randomized, parallel group study large enough (n = 9193) to have sufficient power (80%) to detect a difference of at least 15% in the incidence of combined cardiovascular morbidity and mortality with use of losartan as opposed to atenolol. The primary end point was a composite of cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke, and investigator reported end points were verified by an expert end point committee. Patients included were men and women aged 55–80 years with previously untreated or treated essential hypertension with mean seated BP in the range of 160–205 mmHg systolic, or 95–115 mmHg diastolic, or both, after 1–2 weeks of receiving placebo and who had not experienced a myocardial infarction or stroke within 6 months and did not require treatment with a β–blocker, angiotensin-converting enzyme inhibitor, or angiotensin-receptor blocker. The trial protocol was approved by all ethics committees concerned, was overseen by an independent data and safety monitoring board and all patients gave written informed consent. Targeting a BP of 140/90 mmHg or lower, double-blind treatment was initiated with losartan, 50 mg, or atenolol, 50 mg, daily and matching placebo of the other agent. Study therapy was up-titrated by addition of hydrochlorothiazide, 12.5 mg, followed by increase in blinded losartan or atenolol to 100 mg/day. If this regimen was not sufficient to reach the target BP, additional open-label upward titration of hydrochlorothiazide and institution of therapy with in most cases calcium channel blocker or additional other medication (excluding β–blockers, angiotensin-converting enzyme inhibitors, and angiotensin-receptor blockers) was added to the treatment regimen [Citation12].
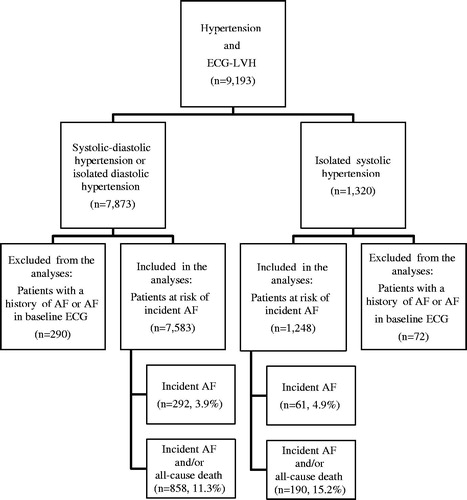
A total of 8831 patients with neither a history of AF or AF on their baseline ECG were included in the present study. Of these, 1248 patients (14.1%) had ISH, a priori defined as systolic BP of 160–205 mmHg and diastolic BP <90 mmHg [Citation8] and 7583 (85.9%) had non-ISH. Characteristics of ISH patients are presented in . Characteristics of the non-ISH patients have been previously published [Citation10].
Table 1. Baseline demographic and clinical characteristics in relation to new-onset atrial fibrillation in 1248 patients with isolated systolic hypertension.
Electrocardiography and end point determination
ECGs were obtained at study baseline, at 6 months, and at yearly follow-up intervals until study termination or patient death as previously reported in detail [Citation4–6]. New-onset AF was a pre-specified secondary end point and was identified by Minnesota coding of annual in-study ECGs at the core laboratory at Sahlgrenska University Hospital/Östra, Göteborg, Sweden [Citation4,Citation12] by experienced readers blinded to clinical information.
Statistical analyses
Data management and statistical analyses were performed by the investigators with SPSS version 22.0 (IBM, Inc, Armonk, NY). Data are presented as mean (SD) for continuous variables and as proportions for categorical variables. Differences in prevalences were compared using χ2 analyses, and differences in mean values were compared using independent-samples t-tests. Associations between SBP during antihypertensive therapy and the occurrence of new-onset AF were analyzed using Cox proportional hazard models [Citation19,Citation20] and based on the intention-to-treat principle. Univariate Cox regression analyses were performed initially to identify important and significant background predictors of AF in the ISH population. The final multivariate models included significant univariate predictors that remained significant in multivariate analyses by using stepwise forward regression. The final model could not include too many predictors due to the number of new-onset atrial fibrillation events. Therefore, Framingham risk score was used to adjust for several cardiovascular risk factors in a propensity score. Baseline and subsequent determinations of SBP were entered as a time-varying covariate in the Cox models. History of coronary heart disease was entered as a standard covariate as it has previously been shown to predict atrial fibrillation [Citation5]. The same multivariate models were used on both ISH and non-ISH patients. In Model 1, in-treatment SBP was adjusted for a treatment group indicator entered as a standard covariate to account for possible effects of treatment with losartan vs. atenolol, and Framingham risk score and history of coronary heart disease included as standard covariates, and in-treatment heart rate and in-treatment ECG-LVH determined by Cornell voltage-duration product entered as time-varying covariates. In Model 2, in-treatment SBP was adjusted for baseline SBP, in addition to the above-mentioned predictors in Model 1. The hazard ratios (HRs) for incident AF were computed for 10 mmHg decrements of in-treatment SBP treated as a continuous variable. The 95% confidence intervals (CI) of HRs were calculated from the estimated coefficients and their standard errors [Citation21], and Wald χ2 statistics and probability values were calculated. In parallel analyses competing risk was analyzed with incident AF and mortality as a combined end point and adjusted for the same variables as in Model 2. In order to test if the effect of reduced SBP on reduced incident AF was significantly different in ISH patients compared to non-ISH patients, interaction analyses were performed using Cox proportional hazard models with all patients and entering ISH status as a categorical covariate and in-treatment SBP as a time-varying covariate and the cross-product of ISH status and time-varying SBP. The p value of this interaction cross-product was used to determine if there was a quantitative interaction, i.e. whether ISH increased or decreased the outcome effect of the reduction in SBP. A two tailed p value of <0.05 was required for statistical significance. All study data reside in a database with the authors.
Table 2. Predictors of new-onset atrial fibrillation in 1248 patients with isolated systolic hypertension.
Results
New-onset AF occurred in 61 patients (4.9%) of 1248 patients with ISH during the 4.8 ± 0.9 years of mean follow-up (). The patients with incident AF were slightly older than those who remained in sinus rhythm, and fewer were treated with losartan, they were taller (though no difference in body mass index), they had higher SBP, pulse pressure and Sokolow-Lyon voltage, and their serum potassium was slightly lower (). New-onset AF occurred in 292 patients (3.9%) of 7583 non-ISH patients during the same mean follow-up time ().
Reductions in SBP were the same in ISH and non-ISH patients whether they had new-onset AF or not (). ISH patients without new-onset AF had SBP values (mean (SD)) at baseline and year 1 to year 5 of follow-up of 174.1 (10.8), 151.9 (17.0), 150.1 (16.4), 148.3 (16.8), 147.7 (16.9) and 148.6 (16.5) mmHg compared to 179.0 (11.5), 153.7 (16.7), 151.1 (20.3), 149.9 (17.8), 147.0 (18.9) and 139.1 (14.8) mmHg in ISH patients with new-onset AF. Non-ISH patients without new-onset AF had SBP values (mean (SD)) at baseline and year 1 to year 5 of follow-up of 174.2 (14.8), 150.0 (16.7), 148.0 (16.2), 146.7 (16.4), 145.4 (15.8) and 144.5 (15.3) mmHg compared to 177.0 (14.2), 152.8 (17.9), 151.0 (17.8), 149.3 (19.9), 145.1 (18.2) and 144.9 (15.4) mmHg in non-ISH patients with new-onset AF.
Figure 2. Development of systolic blood pressure (, upper panel) and diastolic blood pressure (, lower panel) in the ISH patients and the non-ISH patients who had incident AF and the patients who did not have incident AF. The y-axes have been truncated.
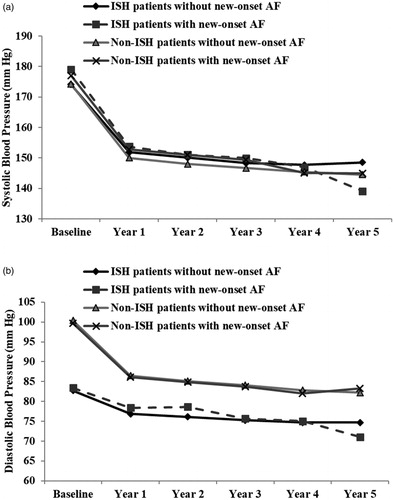
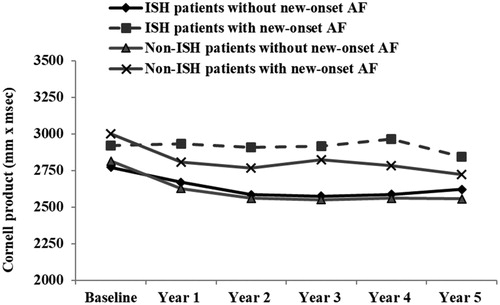
Diastolic blood pressures were the same in patients with and without new onset AF (). ISH patients without new-onset AF had diastolic BP values (mean (SD)) at baseline and year 1 to year 5 of follow-up of 82.7 (5.8), 76.9 (8.9), 76.1 (8.8), 75.2 (9.3), 74.7 (9.0) and 74.7 (8.7) mmHg compared to 83.4 (4.8), 78.4 (8.5), 78.7 (9.9), 75.7 (10.4), 75.1 (9.6) and 71.1 (9.2) mmHg in ISH patients with new-onset AF. Non-ISH patients without new-onset AF had DBP values (mean (SD)) at baseline and year 1 to year 5 of follow-up of 100.4 (6.4), 86.5 (8.5), 85.2 (8.2), 84.1 (8.5), 82.8 (8.5) and 82.2 (8.1) compared to 99.7 (6.1), 86.1 (8.8), 84.9 (8.3), 83.7 (8.8), 82.1 (9.6) and 83.3 (8.9) mmHg in non-ISH patients with new-onset AF. Cornell voltage product as a measure of ECG-LVH was however higher throughout the study in both ISH and non-ISH patients who developed new-onset AF compared to the patients who remained without AF ().
Figure 3. Cornell product as indicator of ECG-LVH at baseline and yearly during the study in the ISH patients and the non-ISH patients with new-onset AF (upper lines) and in patients without new-onset AF during the study (lower lines). The y-axis has been truncated.
We analyzed univariate predictors of new-onset AF in the ISH patients (). There was a 4% increased risk of new-onset AF per 1 mmHg rise in SBP at baseline. Furthermore, height, baseline Sokolow-Lyon voltage, potassium, treatment allocation, time-varying heart rate and age were significant univariate predictors of incident AF, but smoking, time-varying Cornell voltage product and gender were not. Univariate predictors of new-onset AF in the non-ISH patients are shown in . We have not elaborated on these in detail as the sample size and thus statistical power were much higher and stronger than in the ISH subset of patients.
Table 3. Predictors of new-onset atrial fibrillation in 7583 patients with non-isolated systolic hypertension.
Table 4. Time dependent (time to event) multivariate Cox regression analyses to assess the risk of incident atrial fibrillation related to in-treatment variation of 10 mmHg in systolic BP in patients with isolated systolic hypertension and non- isolated systolic hypertension.
The effect of reduction of in-treatment SBP is further explored in multivariate analysis (). While the reduction in new-onset AF in ISH patients was borderline significant in univariate analysis (p = 0.070), and when adjusted for treatment effect only (p = 0.066), it was highly significant when adjusted for baseline SBP and in the multivariate model 2 (p = 0.008 for both). Lower in-treatment SBP was associated with a 17% risk reduction for new-onset AF per 10 mmHg decrease in SBP in ISH patients (p = 0.008), and 9% risk reduction (p = 0.006) in non-ISH patients, independent of treatment modality, baseline risk factors, baseline SBP and in-treatment heart rate and ECG-LVH.
There was a significant interaction (p = 0.041) in favor of stronger effect of SBP reduction on preventing new onset AF in ISH vs. non-ISH patients.
New-onset AF or death occurred in 190 (15.2%) of the ISH patients and in 858 (11.3%) of the non-ISH patients (). Lower in-treatment SBP was associated with lower risk of new-onset AF or death in the non-ISH patients (p < 0.001, Model 2), however the effect of lower in-treatment SBP on the combined endpoint new-onset AF and death was only borderline significant in the ISH patients (p = 0.087) in Model 2 which included adjustment for baseline SBP ().
Table 5. Time dependent (time to event) multivariate Cox regression analyses to assess the risk of the combined end point of incident atrial fibrillation or all-cause mortality related to in-treatment variation of 10 mmHg in systolic BP in patients with isolated systolic hypertension and non-isolated systolic hypertension.
Discussion
New-onset AF occurred in 61 (4.9%) of 1248 patients with ISH and ECG-LVH. Lower in-treatment SBP was associated with a 17% risk reduction for new-onset AF per 10 mmHg decrease in SBP, independent of treatment modality, baseline risk factors, baseline SBP and in-treatment heart rate and ECG-LVH in patients with ISH. There was a significant interaction in favor of stronger impact of SBP reduction in preventing incident AF in ISH vs. non-ISH patients. When taking death and competing risk into account the overall findings confirmed the treatment benefits on preventing new-onset AF both in patients with ISH and in patients with non-ISH.
Our findings suggest that reduced SBP per se may reduce the incidence of AF in patients above 55 years of age with ISH and ECG-LVH. The impact of SBP reduction in preventing incident AF is even slightly stronger in patients with ISH compared to non-ISH patients recruited from the same population. These finding are in line with our previous findings showing benefits of SBP lowering therapy and regression of ECG-LVH on preventing cardiovascular mortality, myocardial infarction, cerebral stroke and heart failure in LIFE study participants with ISH [Citation8–10]. The patients who develop new-onset AF and other complications remained with higher Cornell voltage product ECG-LVH throughout the study as depicted in .
Atrial fibrillation is associated with increased risk of cardiovascular morbidity and mortality. It is important to identify modifiable risk factors as both men and women have an approximate 25% overall lifetime risk of AF [Citation22]. To our knowledge this is the first study to report an effect of lower in-treatment SBP and reduced new-onset AF in patients with ISH and ECG-LVH.
AF is the most prevalent sustained cardiac arrhythmia and the prevalence is increasing [Citation23]. In the Rotterdam study, the prevalence of AF varied from 0.7% in the age group 55–59 years to 17.8% in those aged 85 years and above [Citation24]. AF incidence increases with age but also with other risk factors [Citation25], including diabetes, obesity, hypertension, LVH, coronary heart disease, congestive heart failure, valvular heart disease and increased left atrial size by echocardiography [Citation26–28]. AF is associated with a four to five fold increased risk of ischemic stroke [Citation29–30] and with a near doubled cardiovascular mortality risk [Citation31]. Prevention of AF is thus of major importance and hypertension is currently the most prevalent, potentially modifiable risk factor, accounting for approximately 14–22% of AF cases [Citation26,Citation32,Citation33].
ISH is closely related to increased pulse pressure, a marker of advanced vascular ageing [Citation34] and arterial stiffness [Citation35,Citation36], which may contribute in the structural and electrical remodeling of the myocardium leading to the development of AF, possibly through increased pulsatile load on the heart and increased left atrial size [Citation37]. Studies have shown that reduced distensibility of large arteries parallel cardiac hypertrophy and remodeling in hypertensive patients [Citation38,Citation39]. Large artery stiffness may increase the work load on the heart similar to volume overload and may thus represent one of the mechanisms by which hypertension leads to eccentric hypertrophy and left atrial enlargement [Citation39]. In a LIFE sub-study, there was a significant correlation between baseline brachial pulse pressure and left atrial size, independent of age, gender and body surface area [Citation40]. Furthermore, there is evidence for linking brachial pulse pressure to microvascular damage in the heart and other target organs, which again may lead to increased peripheral resistance and blood pressure, further increasing arterial stiffness and central pulse pressure. Increased central pulse pressure may then further damage small arteries and lead to LVH [Citation41]. Studies have found brachial pulse pressure to be a powerful predictor of cardiovascular morbidity and mortality [Citation42–49], and the predictive effect increases with age [Citation46–48]. The present study evaluated ISH in parallel with brachial pulse pressure and not central pulse pressure. Non-invasive central pulse pressure has been shown to better predict cardiovascular outcomes than brachial pulse pressure, and to be closer associated with extent of atherosclerosis (carotid plaque burden and intimal-medial thickness, and vascular mass) [Citation50].
Some strengths and limitations of the present study should be mentioned. This is a post hoc analysis of data collected prospectively in a randomized clinical trial. Atrial fibrillation was a pre-specified secondary end point and the ISH patients defined as a subgroup of patients of special interest. In the present analysis a strict and conservative approach was used in as much as new-onset AF was ascertained on yearly study ECGs. This ensured objective documentation of all incident cases of AF, however, the true incidence may have been underestimated by missing possible cases of paroxysmal AF. The patients recruited in the LIFE study all had ECG-LVH and results cannot automatically be extrapolated to hypertensive patients at lower risk. Also we excluded patients with AF at baseline known to have especially high cardiovascular risk and strong benefit of SBP reduction. Such exclusion of study participants confounded the relationship between the reduction of SBP and all-cause mortality in the ISH group due to the higher prevalence of AF at baseline in this group.
Further, we investigated a fairly homogenous study population of mostly Caucasian patients with LVH. Systolic blood pressure at baseline when patients were in the untreated state was rather strongly predicting new-onset AF despite quite aggressive treatment and lowering of SBP by about 30 mmHg throughout. Blood pressures were taken standardized and regularly which allowed calculation of in-treatment SBP, also strongly predicting less new-onset AF. Certainly, statistical interference happened between SBP at baseline, changes in SBP during treatment and achieved in-treatment SBP during the study. However, despite these rather complicated relations between various SBPs, adjusted for in multivariate models, quite strong relationships appeared between SBP and risk for new-onset AF and benefits of treatment.
Conclusions
The impact of in-treatment SBP in preventing incident AF was stronger in patients with ISH compared to non-ISH patients in the same population. This finding is in line with our previous findings showing benefits of BP lowering therapy and regression of ECG-LVH on preventing cardiovascular mortality, myocardial infarction, cerebral stroke and heart failure in LIFE study participants with ISH. Overall our findings are principally the same in ECG-LVH hypertensive patients with and without ISH.
Disclosure statement
The LIFE study (Losartan Intervention For Endpoint reduction in hypertension) was originally sponsored by Merck and Co. Inc., Whitehouse Station, NJ, USA. Outside the present work SEK has received ad hoc honoraria for lecturing from Bayer, Merck KGaA, Merck & Co., Sanofi, and Takeda, and honoraria from Takeda for study committee work within the past 3 years. Richard B. Devereux has received honoraria and grant from Merck. Kristian Wachtell has received honoraria from Merck. The other authors report no conflicts.
Additional information
Funding
References
- Kannel WB, Gordon T, Schwartz MJ. Systolic versus diastolic blood pressure and risk of coronary heart disease. The Framingham study. Am J Cardiol. 1971;27:335–346.
- Kannel WB, Wolf PA, McGee DL, et al. Systolic blood pressure, arterial rigidity, and risk of stroke. The Framingham Study. JAMA. 1981;245:1225–1229.
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. JAMA 1991;265:3255–3264.
- Dahlöf B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention for Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002;359:995–1003.
- Wachtell K, Lehto M, Gerdts E, et al. Angiotensin II receptor blockade reduces new-onset atrial fibrillation and subsequent stroke compared to atenolol: the LIFE Study. JACC 2005;45:712–719.
- Okin PM, Wachtell K, Devereux RB, et al. Regression of electrocardiographic left ventricular hypertrophy and decreased incidence of new-onset atrial fibrillation in hypertensive patients: the LIFE study. JAMA 2006;296:1242–1248.
- Okin PM, Wachtell K, Kjeldsen SE, et al. Incidence of atrial fibrillation in relation to changing heart rate over time in hypertensive patients: the LIFE study. Circ Arrhythm Electrophysiol. 2008;1:337–343.
- Kjeldsen SE, Dahlöf B, Devereux RB, et al. Effects of losartan on cardiovascular morbidity and mortality in patients with isolated systolic hypertension and left ventricular hypertrophy. A losartan intervention for endpoint reduction (LIFE) substudy. JAMA 2002;288:1491–1498.
- Larstorp ACK, Okin PM, Devereux RB, et al. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive therapy and the prediction of major cardiovascular events in patients with isolated systolic hypertension. J Hum Hypertens. 2011;25:178–185.
- Larstorp ACK, Okin PM, Devereux RB, et al. Reduced ECG-LVH during antihypertensive therapy is associated with less new-onset heart failure and mortality in patients with isolated systolic hypertension. The LIFE study. Am J Hypertens. 2012;25:1101–1109.
- Larstorp ACK, Ariansen I, Gjesdal K, et al. Association of pulse pressure with new-onset atrial fibrillation in patients with hypertension and left ventricular hypertrophy: the Losartan Intervention For Endpoint (LIFE) reduction in hypertension study. Hypertension. 2012;60:347–353.
- Dahlöf B, Devereux R, de Faire U, et al. The Losartan Intervention for Endpoint reduction (LIFE) in Hypertension study: rationale, design, and methods. The LIFE Study Group. Am J Hypertens. 1997;10:705–713.
- Dahlöf B, Devereux RB, Julius S, et al. Characteristics of 9194 patients with left ventricular hypertrophy: the LIFE study. Losartan intervention for endpoint reduction in hypertension. Hypertension. 1998;32:989–997.
- Kjeldsen SE, Dahlöf B, Devereux RB, et al. Lowering of blood pressure and predictors of response in patients with left ventricular hypertrophy: the LIFE study. Losartan Intervention for Endpoint. Am J Hypertens. 2000;13:899–906.
- Molloy TJ, Okin PM, Devereux RB, et al. Electrocardiographic detection of left ventricular hypertrophy by the simple QRS voltage-duration product. J Am Coll Cardiol. 1992;20:1180–1186.
- Okin PM, Roman MJ, Devereux RB, et al. Electrocardiographic identification of increased left ventricular mass by simple voltage-duration products. J Am Coll Cardiol. 1995;25:417–423.
- Okin PM, Roman MJ, Devereux RB, et al. Time-voltage QRS area of the 12-lead electrocardiogram: detection of left ventricular hypertrophy. Hypertension. 1998;31:937–942.
- Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J. 1949;37:161–186.
- Cox DR. Regression models and life-tables. J R Stat Soc B. 1972;34:187–220.
- Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. New York (NY): John Wiley & Sons; 1980.
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481.
- Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation 2004;110:1042–1046.
- Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics-2012 update: a report from the American Heart Association. Circulation 2012;125:e2–e220.
- Heeringa J, van der Kuip DA, Hofman A, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27:949–953.
- Khairallah F, Ezzedine R, Ganz LI, et al. Epidemiology and determinants of outcome of admissions for atrial fibrillation in the United States from 1996 to 2001. Am J Cardiol. 2004;94:500–504.
- Benjamin EJ, Levy D, Vaziri SM, et al. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–844.
- Psaty BM, Manolio TA, Kuller LH, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation 1997;96:2455–2461.
- Wang TJ, Parise H, Levy D, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA 2004;292:2471–2477.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983–988.
- Go AS. The epidemiology of atrial fibrillation in elderly persons: the tip of the iceberg. Am J Geriatr Cardiol. 2005;14:56–61.
- Benjamin EJ, Wolf PA, D’Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998;98:946–952.
- Aksnes TA, Flaa A, Strand A, et al. Prevention of new-onset atrial fibrillation and its predictors with angiotensin II-receptor blockers in the treatment of hypertension and heart failure. J Hypertens. 2007;25:15–23.
- Huxley RR, Lopez FL, Folsom AR, et al. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation 2011;123:1501–1508.
- Aviv A. Hypothesis: pulse pressure and human longevity. Hypertension. 2001;37:1060–1066.
- Darne B, Girerd X, Safar M, et al. Pulsatile versus steady component of blood pressure: a cross-sectional analysis and a prospective analysis on cardiovascular mortality. Hypertension 1989;13:392–400.
- Franklin SS, Sutton-Tyrrell K, Belle SH, et al. The importance of pulsatile components of hypertension in predicting carotid stenosis in older adults. J Hypertens. 1997;15:1143–1150.
- Vaziri SM, Larson MG, Lauer MS, et al. Influence of blood pressure on left atrial size. The Framingham Heart Study. Hypertension. 1995;25:1155–1160.
- Bouthier JD, De Luca N, Safar ME, et al. Cardiac hypertrophy and arterial distensibility in essential hypertension. Am Heart J. 1985;109:1345–1352.
- Boutouyrie P, Laurent S, Girerd X, et al. Common carotid artery stiffness and patterns of left ventricular hypertrophy in hypertensive patients. Hypertension. 1995;25:651–659.
- Gerdts E, Papademetriou V, Palmieri V, et al. Correlates of pulse pressure reduction during antihypertensive treatment (losartan or atenolol) in hypertensive patients with electrocardiographic left ventricular hypertrophy (the LIFE study). Am J Cardiol. 2002;89:399–402.
- Laurent S, Briet M, Boutouyrie P. Large and small artery cross-talk and recent morbidity-mortality trials in hypertension. Hypertension. 2009;54:388–392.
- Benetos A, Rudnichi A, Safar M, et al. Pulse pressure and cardiovascular mortality in normotensive and hypertensive subjects. Hypertension. 1998;32:560–564.
- Domanski MJ, Davis BR, Pfeffer MA, et al. Isolated systolic hypertension: prognostic information provided by pulse pressure. Hypertension. 1999;34:375–380.
- Millar JA, Lever AF. Implications of pulse pressure as a predictor of cardiac risk in patients with hypertension. Hypertension 2000;36:907–911.
- Franklin SS, Khan SA, Wong ND, et al. Is pulse pressure useful in predicting risk for coronary heart disease? The Framingham Heart Study. Circulation 1999;100:354–360.
- Vaccarino V, Berger AK, Abramson J, et al. Pulse pressure and risk of cardiovascular events in the systolic hypertension in the elderly program. Am J Cardiol. 2001;88:980–986.
- Glynn RJ, Chae CU, Guralnik JM, et al. Pulse pressure and mortality in older people. Arch Intern Med. 2000;160:2765–2772.
- Khattar RS, Swales JD, Dore C, et al. Effect of aging on the prognostic significance of ambulatory systolic, diastolic, and pulse pressure in essential hypertension. Circulation 2001;104:783–789.
- Fyhrquist F, Dahlöf B, Devereux RB, et al. Pulse pressure and effects of losartan or atenolol in patients with hypertension and left ventricular hypertrophy. Hypertension. 2005;45:580–585.
- Roman MJ, Devereux RB, Kizer JR, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension 2007;50:197–203.