Abstract
Aims: Non-adherence to medication is a key challenge in treatment of hypertensive patients. Directly Observed Therapy prior to ambulatory blood pressure measurement (DOT-HTN) is relatively new in hypertension research and knowledge about its use and patients’ perception of such control is warranted.
We aimed to investigate DOT-HTN in relation to blood pressure control, procedural safety and patients’ perception.
Methods and results: Twenty patients with uncontrolled hypertension (daytime systolic ambulatory blood pressure measurement (ABPM) ≥135 mm Hg) were randomized to intervention with DOT-HTN and a visual analogue scale (VAS) assessment if they found DOT-HTN problematic (10 cm = very problematic), or to standard ABPM. They were followed for 2–4 weeks.
There were no differences in baseline characteristics. Despite no difference in daytime systolic ABPM (p = 0.67) two patients were suggested to be non-adherent after DOT-HTN with reductions in daytime systolic ABPM of 18 and 22 mm Hg, respectively. No post DOT-HTN adverse reactions were reported. VAS assessment indicated that the patients had no problem being controlled (VAS median 0.30 cm (0.0–2.6)), however interesting comments and observed behaviour questioned the reliability of the patient-reported VAS in 38% of patients.
Conclusions: Two of eight patients seemed to be non-adherent after DOT-HTN. Descriptive findings suggested reluctance towards control with DOT-HTN not captured by the VAS assessment. No DOT-related medical adverse-effects were reported.
Introduction
Non-adherence has been a challenge in medicine since Hippocrates, and even referred to as an intrinsic property of human beings [Citation1]. Estimated 19–86% of hypertensive patients are reported non-adherent with objective adherence control methods, such as therapeutic drug monitoring (TDM) and directly observed therapy prior to ambulatory blood pressure measurement (DOT-HTN) [Citation2].
Objective adherence control with TDM involves blood or urine samples being analyzed with high performance liquid chromatography coupled with mass spectrometry for drugs and drug metabolites [Citation3]. Recent observational studies have, by use of TDM revealed presumed treatment resistant patients as completely non-adherent in 12–53% of cases [Citation4–10]. Availability and prices of TDM varies between countries and regions, which may favor DOT-HTN when applied at hypertension specialist departments with access to ambulatory blood pressure measurement devices.
In the 1990s directly observed therapy short course (DOTS) originated as a tuberculosis treatment strategy [Citation11] with the purpose of ensuring and monitoring the intake of medication, as well as monitoring risk factors such as toxicity and drug resistance. Directly observed therapy has since been applied in the treatment of various chronic conditions such as hemodialysis, diabetes, chronic hepatitis C infection, major mental illness, as well as in hypertension. DOT-HTN entails health personnel observed intake of patients’ antihypertensive medication prior to ambulatory blood pressure measurement (ABPM) [Citation12,Citation13]. It is a single-day or one-time method to investigate adherence status during visits to a hypertension specialist department, suitable to demonstrate to both patient and treating physician, that blood pressure may decrease if treatment plan is followed. It has been used for several consecutive days as well, in cases of hospitalization of patients with severe uncontrolled and resistant hypertension. No consensus exists on either which magnitude of decrease in blood pressure after DOT-HTN is considered as indication of non-adherence nor on the procedure itself or its safety [Citation13]. We know little about how health personnel negotiate the method, and more importantly to what degree the patients accept it. We aimed to investigate DOT-HTN as a one-time control of adherence in patients with uncontrolled hypertension referred to a specialist hypertension clinic. We hypothesized a priori that there would be a difference in blood pressure after DOT-HTN in favor of the intervention group, and that patients if revealed non-adherent after DOT, would react negatively to the method.
Material and methods
Trial design
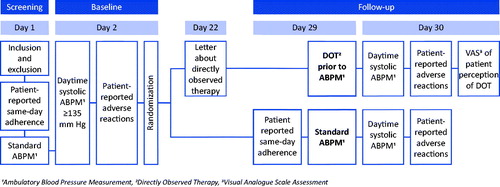
We conducted a parallel assignment open-label randomized controlled trial at an outpatient specialist hypertension clinic. Subjects with uncontrolled ambulatory blood pressure were randomized either to a control group with follow-up measurements of standard care ABPM, or to an intervention group with DOT-HTN. Follow-up was scheduled at 3 weeks ±7 days after baseline. The study was approved by The National Committee for Research Ethics in Norway (2015/159) and the institutional research committee at the University of Oslo. All patients provided written informed consent.
Participants
From February 2015 to July 2018, patients from all categories of uncontrolled hypertension, referred for ABPM from general practitioners, external specialists as well as internal referrals, were assessed for eligibility by screening of referral letters and medical records only (). Inclusion criteria were defined as subjects of both sex, ≥18 years of age, residing in the hospital region, having uncontrolled hypertension defined as daytime systolic ABPM ≥135 mm Hg despite ≥2 antihypertensive drugs and capability of speaking and reading Norwegian. Exclusion criteria were critical illness under active treatment, known atrial fibrillation or heart valve stenosis, myocardial infarction, angina pectoris or stroke during the past 6 months, known severe renal impairment (eGFR <30 mL/min/1.73 m2), history of DOT prior to ABPM or ongoing participation in other clinical trials.
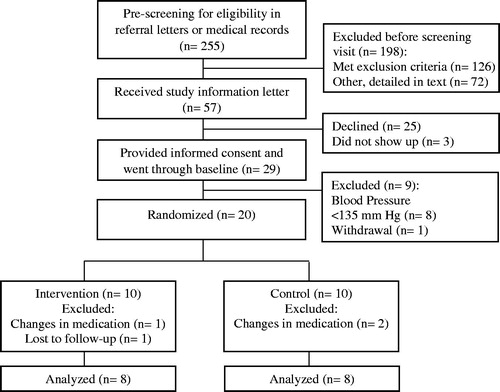
Figure 1. CONSORT Flow Diagram of population, participant eligibility, randomization, and follow-up during the course of the trial.
All presumably eligible patients were informed that the intervention involved close monitoring of treatment, however, no detailed specifications related to DOT-HTN were provided before seven days prior to follow-up visit.
Screening
All consenting and presumably eligible patients underwent a screening visit (). They were asked if they had taken their medication that day, onward referred to as same-day adherence. ABPM was measured using a Spacelabs Healthcare model 90217-1Q device (Spacelabs Medical, Inc. 5150 220th Ave SE Issaquah, WA 98029, USA), preceded by fitting of appropriate cuff according to measurement of upper arm circumference. Daytime measurements were by default carried out from 6 am to 10 pm, and subsequently adjusted to the patients’ individual reporting of daytime period. ABPM was measured according to European Hypertension guidelines [Citation14]. Data on demographics, comorbidity and medication status were collected.
Baseline
At baseline on delivery of the ABPM device, patient-reported adverse reactions were collected and eligibility checked. Patients with normalized daytime ABPM were excluded at this point, and those with uncontrolled ABPM were randomized in 5 blocks of 4 with allocation ratio 1:1 [Citation15].
Follow-up
Prior to follow-up, patients in the intervention group were informed in a letter to meet medication-fasting and to bring their medication in original packaging, for the purpose of taking them in front of the investigator (). The atmosphere during follow-up visits was planned to be equally relaxed in both groups to minimize the impact of control on intervention patients. Patients in the intervention group were asked to dispense their brought medications from original packaging. DOT-HTN was performed by the investigator observing the patients’ intake of prescribed medication (morning dose) followed by mounting of ABPM device. To further ensure the absorption of medication, the investigator stayed with the patients for approximately 30 min. The ABPM device was controlled twice and the patients were asked to stay on the premises for a minimum of 2 h post DOT-HTN enabling safety documentation of possible adverse reactions [Citation16].
At device delivery the intervention patients were asked by an outpatient nurse to complete a self-reported visual analogue scale assessment (VAS) [Citation17,Citation18] of their perception of having DOT-HTN, by marking a 10 cm line: 0 signifying ‘not problematic at all’ and 10 signifying ‘very problematic’. Patients with continued uncontrolled BP or a decrease in BP indicating non-adherence to treatment, were scheduled for further post-study medical follow-up.
Statistical and descriptive analyses
Statistical analyses were performed using IBM SPSS Statistics version 25. Non-parametric tests were applied to determine any significant differences between groups at baseline. The data were presented as median (min-max). Mann-Whitney U test was used to investigate differences in outcome variables between groups from baseline to follow-up with two-sided tests at the 5% level of significance. Descriptive analyses were performed on the entire baseline sample (n = 20) with emphasis on the patient perspective, i.e. patient-reported same-day adherence, patient-reported perception of being controlled with DOT-HTN, patient-reported medicinal side effects, and safety of the procedure indicated by adverse reactions ≤2 h post DOT-HTN.
Results
During the study period a total of 255 patients were pre-screened (). Of the 126 patients meeting one or more exclusion criteria 49 (39%) had co-morbidity, 37 (29%) had normalized blood pressure and 23 (19%) were treated with <2 antihypertensive medications, 5 (4%) were non-residents to the hospital region and 1 (<1%) was pregnant. 72 patients were excluded mainly due to scheduling with lack of clinical and/or study personnel resources or due to competing activities. There were no significant differences in baseline characteristics between groups ().
Table 1. Baseline characteristics.
Nine (45%) patients included in the main statistical analysis were referred due to difficult-to-control hypertension (). The rest of the patients were uncontrolled hypertensive patients referred for 24-h ABPM.
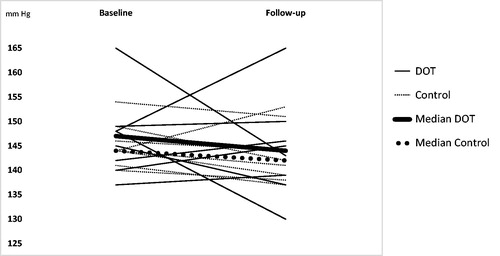
Follow-up time in the intervention group and the control group were median (min–max) 15 (14–28) days and 16 (14–26) days respectively. In the intervention group (n = 8) baseline and follow-up median (min–max) daytime systolic ABPM were 147 mm Hg (137–165) and 144 mm Hg (130–165), respectively (). In the control group (n = 8) baseline and follow-up median (min–max) daytime systolic ABPM were 144 mm Hg (140–154) and 142 mm Hg (137–153), respectively (). A Mann-Whitney U test revealed no significant difference in change in median (min–max) daytime systolic ABP (mm Hg) between baseline and follow-up between intervention patients (2 (−22–17) mm Hg) and control patients (−3 (−7–9) mm Hg), U = 28, z = −.42, p = 0.674, r = 0.11.
Figure 3. Individual and median changes in daytime ambulatory systolic blood pressure from baseline to follow-up in both groups (n= 16). The y-axis has been truncated.
Additional descriptive analyses of patient-reported adherence and perception of DOT-HTN measured with visual analogue scale assessment
Same-day adherence at baseline (n = 20) was confirmed by seven patients in the intervention group and nine patients in the control group. Perception of DOT-HTN measured with VAS (n = 7) showed a median (min-max) of 0.30 cm (0.0–2.6), where VAS 10 cm indicated ‘very problematic’. Two patients in the intervention group were suspected to be non-adherent after DOT-HTN at follow-up, both being referred due to difficult-to-control hypertension. Of these, one patient declined same-day adherence at baseline, presented with a decrease in mean daytime systolic ABPM of 18 mm Hg after DOT-HTN at follow-up, and a reported perception of DOT-HTN as not problematic (VAS, 0.3 cm). The other patient confirmed same-day adherence at baseline with a subsequent decrease in mean daytime systolic ABPM of 22 mm Hg at follow-up. This patient did not want to provide a VAS measurement. One patient in the control group with difficult to control hypertension, confirmed same-day adherence at baseline and presented with a baseline daytime ambulatory systolic and diastolic blood pressure of 186/98 mm Hg. During a safety consultation with a physician, the patient admitted to non-adherence to diuretics. For safety reasons additional antihypertensive drugs were prescribed with a subsequent fall in mean daytime systolic and diastolic ABPM of 27 and 16 mm Hg respectively at follow-up, and hence removal from statistical endpoint analyses.
One patient in the intervention group confirming same-day adherence at baseline, did not bring antihypertensive medication in original packaging to the visit with DOT-HTN in spite of being otherwise instructed in the letter prior to follow-up, and claimed to have swallowed the medication when investigator turned to find a glass of water. The patient reported perception of DOT-HTN as not problematic (VAS, 0.10 cm). One patient after providing a VAS assessment of 2.6 cm added: ‘why do you ask me this, I am an adult person?’ The patient in the intervention group who was lost to follow-up did not meet or respond to attempts of contact from the investigator after receiving the letter with information about DOT-HTN.
Patient-reported medicinal adverse reactions and safety of DOT-HTN
Patient-reported medicinal adverse reactions were reported in a patient diary on the day of ABPM device delivery after baseline (n = 19) and follow-up (n = 17). Three intervention- and two control group patients reported adverse reactions to antihypertensive medications at baseline, of which two of the intervention patients and one of the control patients repeated a similar report at follow-up. There were no cases of adverse reactions ≤2 h post-DOT-HTN in the intervention group (n = 8).
Discussion
We conducted a randomized controlled trial investigating and characterizing DOT-HTN as an intervention in regards to effect on blood pressure as well as procedural safety and patients perception. We also sought to gather experience on how health personnel negotiate the method, but did not provide a tool to measure this, which in hineside can be seen as a limitation. Our sample consisted of mainly middelaged caucasian men treated with hypertension for approximately 10 years, half of them referred to the specialist department due to difficult-to-control hypertension. More than half of the sample had co-morbidity, e.g. diabetes or cardio- or cerebrovascular disease and they were mainly non-smokers. We found no significant effect of DOT-HTN on blood pressure and there were no adverse reactions to the procedure.
Interestingly more than a third of the patients either displayed behavior or added comments, that could indicate a tendency to sceptisism towards being controlled with directly observed therapy, however not captured by our visual analogue scale assessment tool. This of course had to be dealt with professionally by the health personnel involved, and is something to be prepared for when negotiating objective adherence control methods like DOT-HTN, as well as TDM. Ethical considerations are mandatory and will be further discussed. In that sense the study provided us with important insight into the complexity of gaining knowledge about patient perception, and questioned the usefulness and validity of a visual analogue scale assessment as an instrument to measure patients´ perception of objective adherence control. Further our additional descriptive analyses made us speculate if it was easier for the patient honestly declining same-day adherence to provide a VAS assessment about perception of DOT-HTN, than for the patient falsely confirming same-day adherence. Being ‘caught’ by health personnel in false declaration of adherence was understandably difficult for the patients, and had to be handled with utmost caretaking for the patient’s dignity. An interpretive phenomenological study of tuberculosis nurses using DOTS, and their relational work [Citation19], stated the importance of the nurses’ ability to balance the dual surveillance-care role, i. e. both providing comfort and being watchful. The similarity between DOTS provided to tuberculosis patients and DOT-HTN provided to hypertensive patients is limited to the purpose of ensuring the intake of medication, however antihypertensive treatment is not mandatory as is that of tuberculosis treatment, and one cannot force a hypertensive patient to ingest his or her medication. When agreeing to treatment however, a patient is trusted to follow the agreed treatment plan, and if treatment goal is not met, the physician is expected to adjust and optimize treatment to meet the goal.
The intervention patient with deviation in the DOT-HTN procedure and yet a reporting of DOT-HTN as non-problematic (VAS, 0.10 cm) was not confronted with the failed procedure, since the purpose of the study in that sense was to observe and describe. A similar situation occurred in another study (2013 personal communication with Elmula FEM), where a patient was pretending to swallow the medication meanwhile keeping the pills hidden in one hand.
Both TDM and DOT-HTN have rightfully been criticized of being ethically problematic [Citation20], especially due to the procedures’ element of control subjected to patients under voluntary medical follow-up. One has to carefully consider how to act and communicate with due respect, with patients subjected to any objective adherence control method, since the trust between caregiver and patient is of great importance to treatment outcome. When applying DOT, the patients could be told that it is done in order to investigate the therapeutic effect of the prescribed antihypertensive regimen. Further, if the post-DOT blood pressure normalizes or show a significant decrease, the physician can enter into a dialogue with the patient about the effect of the prescribed medication, when administered correctly. If on the other hand the blood pressure remains uncontrolled, the physician can evaluate and adjust the regimen in cooperation with the patient. The World Health Organization defines adherence as ‘…the extent to which a person’s behaviour – taking medication, following a diet, and/or executing lifestyle changes – corresponds with agreed recommendations from a health care provider.’ [Citation21], others as ‘…the process by which patients take their medications as prescribed, composed of initiation, implementation, and discontinuation.’ [Citation1]. When we informed our patients about DOT-HTN in a letter prior to follow-up, one patient did not meet. Nine patients completed DOT-HTN at follow-up, but one did not follow instructions. All in all, over one third of patients displayed contradictory behaviour compared to their VAS assessment. The complexity of non-adherence is mirrored in the multiple methods applied over the past fifty years. The main concern in the 1970s was the impact of non-adherence on the results of clinical trials [Citation1]. Since then, single-factor indirect adherence assessment methods, such as electronic pillboxes [Citation22] and questionnaires [Citation23] have shown inferiority to multifactorial approaches such as optimization of drug regimen, educational interventions, self-monitoring, motivational interviews, refill-reminders etc [Citation21,Citation24,Citation25].
Comparing our trial with other hypertension studies investigating DOT was challenging due to differences in methodology [Citation13,Citation26,Citation27]. Hameed et al. [Citation28] reported retrospectively, and without prior consent, on patients (n = 50) with presumed treatment resistant hypertension referred to a DOT clinic, in comparison to our consenting prospectively randomized sample (n = 20) with 45% difficult-to-control hypertensive patients. They found 50% of patients to be non-adherent after DOT-HTN compared to our 25%. Major methodological differences between the two studies make a comparison difficult, as well as the fact that we in our RCT asked participants for willingness to participate in a study involving close monitoring of treatment, hence may have lost some deliberately non-adherent patients, or, due to our exclusion criteria, may have lost patients with more co-morbidities. Polypharmacy is frequent in patients with high comorbidity and data has shown a close relationship between the number of drugs prescribed and non-adherence [Citation29]. A study [Citation16] in patients with difficult to control hypertension using three or more antihypertensive drugs, revealed non-adherence using DOT-HTN in 39%, and interpreted a normalization of daytime ABPM after DOT-HTN as proof of non-adherence.
Hameed et al. [Citation28] used, in the absence of a defined cut-off value in the literature an arbitrary cut-off of ≥5 mm Hg difference in systolic BP to determine non-adherence. From a patient perspective one could question the reasonability of a cut-off value as low as ≥5 mm Hg, since a predicate of non-adherence might be difficult for the patient to accept, and might jeopardize the important trust between doctor and patient.
In the present study no cases of hypotension were seen in contrast to other studies and case reports [Citation16,Citation30].
Limitations
Our study has several limitations. One major limitation is the limited sample size. 25 of 57 (44%) of patients who received an invitation letter to participate in the study, declined participation. Possible explanations to such a high number of patients being negative to participation could be resistance to undergo two ABPM only 2–4 weeks apart, or that they did not want close monitoring of treatment as informed of prior to consent. The patient who withdrew consent after baseline stated the repeated ABPM as a cause for withdrawal. The high number of non-eligible patients after pre-screening of referral letter or medical records might have been increased by less restrictive exclusion criteria, under the argument that no participant would be exposed to any additional risk, since no changes in medications were undertaken, and since we merely wanted to observe their intake of doctor-patient-agreed prescribed medications. However research has revealed both patient declared adherence [Citation21] and physician’s ability to judge patient’s adherence [Citation31] as questionable, making it unsafe and unethical to actually ‘expose’ patients with additional vulnerability in terms of the co-morbidities listed in our exclusion criteria, to their medications if they are not used to take them.
A one-time control with DOT-HTN, as we investigated in this study, does not provide information on the patient’s long-term adherence, which is a known obstacle in all research on adherence. The main difference between a single DOT-HTN intervention as we did, and a repeated DOT intervention, in tuberculosis and other diseases including hypertension, is that a single intervention is a one-time status or snap-shot of a given treatments therapeutic effect on the blood pressure.
We had inadequate statistical power to make valid conclusions about the changes in blood pressure, hence keeping the null-hypothesis of no difference, with the risk of a type 2 error. Main statistical analysis was performed on only eight patients in each group, due to post baseline changes in medication in 3 (15%) of the randomized patients, and one lost to follow-up. Medication changes could have been avoided had all patients been screened with ABPM in order to adjust and optimize medication regimen, prior to baseline and follow-up ABPM [Citation16].
We asked the patients about same-day adherence and not adherence in general, which was a limitation to the conclusion about adherence in this study. However, four (20%) patients answered ‘no’ to same-day adherence, i.e. saying that they would take the medication after the study visit. The fact that we asked the patients about same-day adherence may have generated a reinforcement of adherence in both groups, leading to white coat adherence at follow-up. It is well known that patients change their behaviour when one starts talking about adherence, or indeed when enrolled in any trial (Hawthorne effect). Had we not asked the patients if they had taken their medication in the morning at baseline, one could speculate that patients would react to a fall in blood pressure by saying that they did not take their medications before the first measurement serving as an explanation for a post-DOT decline in blood pressure.
Our choosing of a VAS assessment tool to measure the patient perception of undergoing adherence control with DOT-HTN most likely failed to capture the patients’ true perception. It might have strengthened reliability of the result had we at least collected the visual analogue scale assessment completely anonymously. In hindsight such complex information would demand either a more complex instrument and/or a qualitatively or mixed methods research approach.
Future research
Further investigation should be focused on the effect of DOT-HTN on behavior changes transformed into better blood pressure control as well as further investigation into the patients’ perception of being controlled. Qualitative or mixed methods research approach may be superior to grasp the patients’ perspective. Ethical considerations are important in the field of drug adherence. Knowing more about how the objective control effects the patients and their reporting, one can plan larger randomized controlled trials with more participants to define a resonable cut-off value defining adherence and hence state the sensitivity and specificity of DOT-HTN as an objective adherence assessment method.
Conclusion
Though no significant finding according to blood pressure, our study provided interesting information about the patients’ perception of DOT-HTN. Additional descriptive findings suggested a reluctance towards DOT-HTN not captured by the VAS assessment. A standarization of the DOT-HTN procedure is warranted.
Acknowledgement
The authors gratefully acknowledge the assistance of Pernille Fabritius Dybwad and Tone Rambjørg Heimstad of the Department of Nephrology, Oslo University Hospital, Ullevaal, and they thank Professor Sverre Erik Kjeldsen of the Department of Cardiology, Oslo University Hospital, Ullevaal, for commenting on this paper.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Burnier M. Vrijens B. Taxonomy of medication adherence: recent developments. In: Burnier M, editor. Drug adherence in hypertension and cardiovascular protection. Cham, Switzerland: Springer International Publishing AG part of Springer Nature; 2018. p. 1–8.
- Eskas PA, Heimark S, Eek Mariampillai J, et al. Adherence to medication and drug monitoring in apparent treatment-resistant hypertension. Blood Press. 2016;25(4):199–205.
- Gundersen POM, Helland A, Spigset O, et al. Quantification of 21 antihypertensive drugs in serum using UHPLC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2018;1089:84–93.
- Tomaszewski M, White C, Patel P, et al. High rates of non-adherence to antihypertensive treatment revealed by high-performance liquid chromatography-tandem mass spectrometry (HP LC-MS/MS) urine analysis. Heart. 2014;100:855–861.
- Jung O, Gechter JL, Wunder C, et al. Resistant hypertension? Assessment of adherence by toxicological urine analysis. J Hypertens. 2013;31:766–774.
- Ceral J, Habrdova V, Vorisek V, et al. Difficult-to-control arterial hypertension or uncooperative patients? The assessment of serum antihypertensive drug levels to differentiate non-responsiveness from non-adherence to recommended therapy. Hypertens Res. 2011;34:87–90.
- Brinker S, Pandey A, Ayers C, et al. Therapeutic drug monitoring facilitates blood pressure control in resistant hypertension. J Am Coll Cardiol. 2014;63:834–835.
- Chung O, Vongpatanasin W, Bonaventura K, et al. Potential cost-effectiveness of therapeutic drug monitoring in patients with resistant hypertension. J Hypertens. 2014;32:2411–2421.
- Pandey A, Raza F, Velasco A, et al. Comparison of Morisky Medication Adherence Scale with therapeutic drug monitoring in apparent treatment-resistant hypertension. J Am Soc Hypertens. 2013;9:420–426.e2.
- Strauch B, Petrak O, Zelinka T, et al. Precise assessment of noncompliance with the antihypertensive therapy in patients with resistant hypertension using toxicological serum analysis. J Hypertens. 2013;31:2455–2461.
- World Health Organization [Internet]. WHO Tuberculosis Programme; Framework for effective tuberculosis control; 1994 [cited 2016 May 2]. Available from: http://apps.who.int/iris/bitstream/10665/58717/1/WHO_TB_94.179.pdf.
- Fadl Elmula FE, Hoffmann P, Fossum E, et al. Renal sympathetic denervation in patients with treatment-resistant hypertension after witnessed intake of medication before qualifying ambulatory blood pressure. Hypertension 2013;62:526–532.
- Hjørnholm U, Aamodt M, Larstorp AC, et al. Directly Observed Therapy in Hypertension (DOT-HTN). In: Burnier M, editor. Drug adherence in hypertension and cardiovascular protection. Cham, Switzerland: Springer International Publishing AG part of Springer Nature; 2018. p. 57–85.
- Williams B, Mancia G, Spiering W, et al. Practice Guidelines for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. Blood Press. 2018;27:314–340.
- Randomization.com [Internet]. [cited 2015 Feb 15]. Available from: http://www.randomization.com/.
- Fadl Elmula FE, Hoffmann P, Larstorp AC, et al. Adjusted drug treatment is superior to renal sympathetic denervation in patients with true treatment-resistant hypertension. Hypertension 2014;63:991–999.
- Haynes M, Patterson DG. Experimental development of the graphic rating method. Psychol Bull. 1921;18:98–99.
- Freyd M. The graphic rating scale. J Educ Psycol. 1923;14:83–102.
- Bender A, Peter E, Wynn F, et al. Welcome intrusions: an interpretive phenomenological study of TB nurses’ relational work. Int J Nurs Stud. 2011;48:1409–1419.
- Hjemdahl P. Ethical aspects of measuring adherence to antihypertensive treatment. In: Burnier M, editor. Drug adherence in hypertension and cardiovascular protection. Cham (Switzerland): Springer International Publishing AG part of Springer Nature; 2018. p. 99–104.
- World Health Organization [Internet]. Adherence to long-term therapies: evidence for action. Geneva: WHO; 2003 [cited 2016 May 2]. Available from: http://www.who.int/chp/knowledge/publications/adherence_report/en/.
- Checchi KD, Huybrechts KF, Avorn J, et al. Electronic medication packaging devices and medication adherence: a systematic review. JAMA 2014;312:1237–1247.
- Morisky DE, Ang A, Krousel M, et al. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertension. 2008;10:348–354.
- Stewart K, George J, Mc Namara KP, et al. A multifaceted pharmacist intervention to improve antihypertensive adherence: a cluster-randomized, controlled trial (HAPPy trial). J Clin Pharm Ther. 2014;39:527–534.
- Berben L, Bogert L, Leventhal ME, et al. Which interventions are used by health care professionals to enhance medication adherence in cardiovascular patients? A survey of current clinical practice. Eur J Cardiovasc Nurs. 2011;10:14–21.
- Heimark S, Eskas PA, Mariampillai JE, et al. Tertiary work-up of apparent treatment-resistant hypertension. Blood Press. 2016;25(5):312–318.
- Miriampillai JE, Eskas PA, Heimark S, et al. Apparent treatment-resistant hypertension – patient-physician relationship and ethical issues. Blood Press. 2017;26:133–138.
- Hameed MA, Tebbit L, Jacques N, et al. Non-adherence to antihypertensive medication is very common among resistant hypertensives: results of a directly observed therapy clinic. J Hum Hypertens. 2016;30:83–89.
- Zarowitz BJ, Stebelsky LA, Muma BK, et al. Reduction of high-risk polypharmacy drug combinations in patients in a managed care setting. Pharmacotherapy. 2005;25:1636–1645.
- Linicus Y, Kindermann I, Helfer AG, et al. Witnessed drug intake before planned denervation–always harmless? Int J Cardiol. 2015;179:125–126.
- Burnier M, Wuerzner G, Struijker-Boudier H, et al. Measuring, analyzing, and managing drug adherence in resistant hypertension. Hypertension 2013;62:218.