Abstract
Purpose: The purpose of this study was to clarify the relationship between decreased sleep quality during the first trimester and a rise in blood pressure during an otherwise normal course of pregnancy in primipara women.
Materials and methods: We recruited 128 pregnant women (primipara) who visited the obstetrics and gynecology clinic for medical examination, of which 89 were longitudinally investigated from the first to the third trimester after obtaining informed consent. A survey was conducted in the first, second, and third trimesters to evaluate sleep quality using the Japanese version of the Pittsburgh Sleep Quality Index (PSQI-J). Patients were assigned to either a good sleep quality group (PSQI-J ≤ 5) or a poor sleep quality group (PSQI-J ≥ 6). Blood pressure was measured using a home blood pressure measurement method. We analyzed the relationship between sleep quality in the first trimester and blood pressure during pregnancy.
Results: The increase in morning systolic blood pressure from first to third trimester was larger in the poor sleep quality group than in the good sleep quality group (7.1 ± 7.0 vs. 3.0 ± 5.6 mmHg, p < .01). Sleep latency (r = 0.38, β = 0.43, p = .02) and sleep disturbances (r = 0.24, β = 0.33, p = .04) in the first trimester affected the increase in systolic blood pressure during pregnancy.
Conclusions: Understanding sleep quality at the beginning of pregnancy can help predict a rise in systolic blood pressure in the third trimester. This emphasizes the importance of sleep education during pregnancy.
Introduction
Reports suggest that blood pressure decreases from the first trimester to the second trimester and is the highest in the third trimester [Citation1]. Elevated blood pressure in pregnancy can have devastating effects on maternal and fetal health, such as causing hypertensive disorders of pregnancy (HDP) [Citation2], premature birth [Citation3], and fetal growth retardation [Citation4]. Pregnant women who develop HDP have an increased risk of cerebrovascular diseases [Citation5] and an elevated mortality rate [Citation6].
Excessive weight gain, primipara [Citation7], age over 35 years when pregnant [Citation8], and obesity [Citation9] are considered risk factors for elevated blood pressure during pregnancy. Therefore, in Japan, it is recommended that all pregnant women undergo regular health check-ups [Citation10]. During these health check-ups, doctors or midwives regularly check maternal health and fetal growth, as well as provide guidance to prevent high blood pressure. Guidance includes advice on diet, exercise, and proper weight control during pregnancy. Thus, a support system for early detection and prevention has been established; however, maternal deaths due to high blood pressure have still been reported [Citation11]. The second cause of maternal death in Japan is cerebral hemorrhage/cerebral infarction, and the prevalence is increasing [Citation11,Citation12].
Recently, sleep duration in the first trimester has been noted as a risk factor for high blood pressure and HDP. Williams et al. reported changes in blood pressure from the first to the third trimester according to sleep duration in the first trimester in 1,272 healthy pregnant women [Citation13]. According to their findings, in pregnant women with a sleep duration of <6 h or ≥10 h, systolic blood pressure (SBP) was elevated by 3.72/4.21 mmHg compared to pregnant women who slept for 9 h. They have also shown that pregnant women who slept for a short or a long time were at an increased risk of developing preeclampsia [Citation13]. In addition, poor sleep quality during pregnancy is related to preterm birth and fetal growth restriction [Citation14,Citation15], as well as abnormalities in the duration of labor and the style of delivery [Citation16].
The average sleep duration of a Japanese woman of reproductive age is 7.05 h [Citation17], which has decreased in the last 10 years and is currently the shortest in the world [Citation18]. According to the National Health and Nutrition Survey in Japan 2016, the ratio of women in their 20 s and 30 s with a sleep duration of <6 h was 39.5% and 36.1%, respectively [Citation18]. Moreover, 29.7% of women in their 20 s and 33.1% of women in their 30 s stated they were not satisfied with the quality of the whole sleep episode [Citation18]. Thus, 30% of Japanese women of reproductive age report poor sleep quality or short sleep duration. Sleep quality in pregnant women decreases over the course of pregnancy, and this sleep quality is worsened in pregnant women who already have poor sleep quality [Citation19].
It is well-known that declining sleep quality in adults is associated with cerebrovascular diseases [Citation20] and hypertension [Citation21]. Epidemiologic investigations have been performed on the deterioration of sleep quality during pregnancy, including associations with small for gestational age (SGA) [Citation14] and preterm delivery [Citation3]. However, there is no clear evidence for the effect of poor sleep quality on blood pressure during pregnancy. The purpose of this study was to clarify how quality of sleep in the first trimester can alter the normal changes in blood pressure during pregnancy.
Materials and methods
Subjects
From August 2014 to May 2016, we conducted a cohort study on pregnant women with a normal course during the first trimester, who visited an obstetrics and gynecology clinic in Kobe, Japan. For this study, the expected date of delivery was confirmed by the obstetrician through ultrasonography, along with an evaluation of the progress of the pregnancy. Exclusion criteria included complications, such as mental disorder, cardiovascular disease, diabetes mellitus, and thyroid disease, pregnancy after infertility treatment, and women who were continuing medication during pregnancy. Exclusion criteria after the start of the survey included abortion, intrauterine fetal death, and transfer to other hospitals (this also included change of facility based on the preference of the pregnant woman).
We collected basic and pregnancy-related information. Weight gain during pregnancy was defined as the increase in weight (kg) from the first to the third trimester. We also collected data on the smoking status (yes/no) of housemates (i.e. all persons living in the house, including non-family members) and family history of hypertension and diabetes, including that of the pregnant woman’s grandparents. Data on birth weight (g), number of gestational weeks (weeks), and delivery style (vaginal/cesarean section) were also collected.
The survey on sleep quality and home blood pressure measurement was conducted during the first trimester (from 10 weeks to 14 weeks), the second trimester (from 22 weeks to 24 weeks), and the third trimester (from 32 weeks to 36 weeks). Selection criteria for the survey subjects included normal primipara pregnant women without complications with a body mass index (BMI) <25.
Sleep quality measurement
We measured sleep quality using the Japanese version of the Pittsburgh Sleep Quality Index (PSQI-J) [Citation22] score. This score: (1) measures average sleep duration in the last month, (2) evaluates quantitative and qualitative information on sleep, (3) can be compared between individuals or groups, (4) is a reliable and valid standardized scale, and (5) is convenient. The PSQI-J consists of seven components of sleep quality. Sleep quality is scored from 0 to 3 points on the Likert scale, and the global score of all seven components ranges from 0 to 21 points. The seven sleep quality components and the corresponding evaluation criteria were as follows: 1) subjective sleep quality (with 0 points being very good, 1 point being quite good, 2 points being rather bad, and 3 points being very bad), 2) sleep latency (0 points for <16 min, 1 point for 16 min to less than 31 min, 2 points for 31 min to less than 61 min, and 3 points for >61 min), 3) sleep duration (0 points for >7 h, 1 point for less than 6 to 7 h, 2 points for 5 h to less than 6 h, and 3 points for <5 h), 4) sleep efficiency (0 points for >85%, 1 point for 75% to less than 85%, 2 points for 65% to less than 75%, and 3 points for <65%), 5) sleep disturbances (0 points for never, 1 point for < once a week, 2 points for 1 to 2 times per week, and 3 points for >3 times a week), 6) use of sleep medication (0 points for never, 1 point for < once a week, 2 points for 1 to 2 times per week, and 3 points for >3 times per week), and 7) daytime dysfunction (frequency of drowsiness during the day and the degree of difficulty in daily life). The cut-off value was 5.5, and a higher score indicated poorer sleep quality.
Home blood pressure measurements
Blood pressure was self-measured by pregnant women using the home blood pressure measurement (HBPM) method [Citation23], after being trained by the researchers regarding the correct way to use the monitor. The blood pressure monitor lent to pregnant women enrolled in this study was an arm-worn digital automatic blood pressure monitor (HEM-7320F; OMRON HEALTHCARE Co., Ltd., Japan), which recorded the measured value and time. Blood pressure was measured in the morning and evening for 5 days in each trimester. The conditions for blood pressure measurement were as follows: sit in a chair, place the cuff sphygmomanometer at the same level as the heart and take the measurement: 1) within an hour of waking up in the morning, 2) before meals, 3) and after urination. Participants were instructed to not take a measurement after meals, exercise, and smoking. The blood pressure data were collected from the memory of the sphygmomanometers. Patients who did not take blood pressure measurements twice daily and did not have 5-day values were excluded from the analysis.
The measured values used for analysis were as follows: (1) mean values of SBP and mean values of diastolic blood pressure (DBP) at each trimester of pregnancy and (2) change in mean SBP/DBP during the course of pregnancy (first to second trimester, first to third trimester, and second to third trimester). When measurements were conducted only once daily, the obtained value was used as the measured value. The hypertension criteria of HBPM was a mean SBP ≥135 mmHg and a mean DBP ≥85 mmHg [Citation23].
Statistical analysis
The data are presented as mean ± standard deviation for normally distributed values, median (interquartile range) for non-normally distributed values, and number (%) for categorical values. For analysis, data that could be longitudinally measured from the first to the third trimester were used. The HBPM used for analysis was the mean SBP/DBP in the mornings and evenings measured during each trimester and the change in HBPM along the course of pregnancy (first to second trimester, first to third trimester, and second to third trimester). A comparison of the characteristics of pregnant women with a PSQI-J ≤ 5 and a PSQI-J ≥ 6 was conducted using the t-test and χ2 test. In addition, comparison of PSQI-J components in each trimester was performed using the Mann–Whitney test. The Friedman test and multiple comparisons were used for comparison between trimester-specific sleep variables in the PSQI-J ≤ 5 and ≥6 groups.
The magnitude of the characteristics affecting the change in HBPM was determined by multiple regression analysis. As an explanatory variable, an attribute that generally affects blood pressure was used [Citation9,Citation22,Citation23]. Furthermore, multiple regression analysis was used to evaluate the magnitude among constitutive PSQI-J factors in the first trimester that affected changes in blood pressure (the PSQI-J consisted of seven components). A p value of <.05 was considered as a significant difference. We used SPSS Statistics version 23.0 (IBM Corp., Armonk, NY, USA) and R version 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria).
Ethical considerations
This research was conducted according to the Declaration of Helsinki. We obtained ethical approval from Kobe University Graduate School of Health Sciences (Approval number 353) and the ethics review committee of Hyogo University of Health Sciences (Approval number 14017). Informed written consent was obtained from all participants in the study.
Results
Characteristics
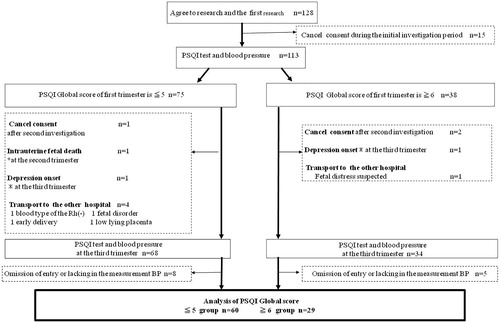
We enrolled 153 primipara pregnant women who fulfilled the selection criteria. Of these women, 128 initially provided written consent and were examined for sleep quality and blood pressure (). Of these, 15 pregnant women withdrew their consent (nine women changed their minds and six chose to deliver at another hospital), leaving 113 respondents in their first trimester. These participants were divided into two groups according to PSQI-J scores [Citation22]. The study population was divided into two groups: those with a PSQI-J ≤ 5 (n = 75, defined as the good sleep quality group), and those with a PSQI-J ≥ 6 (n = 38, defined as the poor sleep quality group). A longitudinal investigation was conducted until the third trimester.
In the good sleep quality group, 15 pregnant women were excluded by the end of the investigation in the third trimester. Eight pregnant women missed an answer in the questionnaires or lacked blood pressure measurements, one withdrew consent, one exhibited symptoms of depression, four were transported to other hospitals (due to blood type Rh(−) [n = 1]; fetal distress [n = 1]; premature birth [n = 1]; low lying placenta [n = 1]), and one experienced intrauterine fetal death. In the poor sleep quality group, nine pregnant women were excluded by the end of the investigation in the third trimester. Five pregnant women missed an answer in the questionnaires or there was no record of blood pressure measurements, two withdrew consent, one exhibited symptoms of depression, and one was transferred to another hospital (fetal distress). Thus, 60 patients in the good sleep quality group and 29 patients in the poor sleep quality group were subject to data analysis.
The characteristics of the 89 enrolled subjects are presented in . The mean age was 30.2 ± 4.9 years, the mean BMI was 20.0 ± 1.9 kg/m2, and the mean weight gain during pregnancy was 8.0 ± 2.0 kg. Sixteen pregnant women had gynecological diseases (uterine myoma, 9; endometriosis, 2; and treatment for infertility, 5), and four had a history of thyroid gland diseases and were stable without treatment. Regarding family history, we noted that 33 (37.1%) were positive for hypertension and 30 (33.7%) for diabetes mellitus. Regarding delivery status, the mean birth weight was 2898.2 g, the mean gestational age was 38.7 weeks, and normal delivery was observed in 96.6% of pregnant women.
Table 1. Participant characteristics.
Alcohol consumption was 69.7% before pregnancy but decreased to about 2% after pregnancy. Smoking was also positive in 16.9% before pregnancy and decreased to about 2% after pregnancy, although the smoking rate of housemates remained the same, at about 35% during pregnancy.
We observed that 6.7% of pregnant women exercised during the first trimester, which increased to 33.7% in the second and third trimesters. Regarding working status, 91% of women worked prior to pregnancy. This figure dropped to 79.8% during the first trimester, 68.5% during the second trimester, and 19.1% during the third trimester. Nine pregnant women demonstrated high blood pressure during labor, which returned to normal after birth. There was no significant difference when comparing the characteristics between the good sleep quality group and the poor sleep quality group. When the global score of the good sleep quality group and the poor sleep quality group in each pregnancy period was compared, we observed that the global score was significantly higher in the poor sleep quality group during the first (p < .01) and second (p < .01) trimesters.
When each component of PSQI-J in the good sleep quality group and the poor sleep quality group was compared, we noted subjective sleep quality (p < .01), sleep latency (p < .01), sleep duration (p < .01), sleep disturbance (p = .01), and daytime dysfunction (p < .01) in the first trimester were significantly higher (worse) in the good sleep quality group than the poor sleep quality group. In the second trimester, sleep latency (p = .04) and sleep duration (p < .01) were significantly higher (worse) in the poor sleep quality group than the good sleep quality group. In the third trimester, only sleep efficiency (p < .01) was significantly higher (worse) in the poor sleep quality group than the good sleep quality group.
Changes in the sleep quality during pregnancy
The median of sleep quality based on the global PSQI-J in the 89 pregnant women in this study was the highest in the third trimester (). This finding indicates that sleep quality was the poorest in the third trimester in our study population, which was consistent with a previous finding [Citation24]. With such a change being observed in all women, pregnant women in the poor sleep quality group, whose decline in sleep quality had started in the first trimester, presented with a different change pattern compared with women in the good sleep quality group (). The global sleep quality in the good sleep quality group became worse from the first to third trimester (3.5 [3–4], 3.5 [3–5], and 5 [3–6], respectively [p < .001]). On the other hand, the global sleep quality in the poor sleep quality group remained poor during pregnancy. Among the PSQI-J components in the good sleep quality group, subjective sleep quality and sleep latency were lowest (best) in the third trimester. Daytime dysfunction was lowest (best) in the first trimester in the poor sleep quality group.
Table 2. Change in sleep quality during pregnancy.
Changes in home blood pressure during pregnancy
The mean SBP in the first, second, and third trimesters was 100.6 mmHg, 100.0 mmHg, and 104.9 mmHg, respectively in the morning (), and 100.8 mmHg, 99.5 mmHg, and 105.1 mmHg, respectively in the evening. The mean DBP in the first, second, and third trimesters was 63.7 mmHg, 61.8 mmHg, and 66.2 mmHg, respectively in the morning, and 61.8 mmHg, 60.6 mmHg, and 65.1 mmHg, respectively in the evening. Home blood pressure in all 89 pregnant women was within the normal range during pregnancy (<125/80 mmHg according to the Japanese Guidelines for the Management of Hypertension 2014) [Citation23], was the lowest in the second trimester, and the highest in the third trimester. We observed that there was no significant difference in the morning or evening home blood pressure between the good sleep quality group and the poor sleep quality group in the first, second, and third trimesters.
Table 3. Changes in home blood pressure during pregnancy.
Comparison of changes in home blood pressure in the good sleep quality group and the poor sleep quality group
When comparing the changes in SBP in the morning between the three trimesters, we observed a significant difference between the good sleep quality group and the poor sleep quality group from the second to the third trimester (3.9 ± 6.3 mmHg and 6.9 ± 5.6 mmHg, p = .03) and from the first to the third trimester (3.0 ± 5.6 mmHg and 7.1 ± 7.0 mmHg, p < .01). The difference in the changes in mean SBP in the evening was only significant from the first to the third trimester (3.2 ± 5.7 mmHg and 6.6 ± 5.9 mmHg, p = .00).
Factors affecting changes in mean SBP
We analyzed the potential factors affecting the changes in mean SBP between the good sleep quality group and the poor sleep quality group using multiple regression analysis, including the following variables: pre-pregnancy BMI, weight gain during gestation, positive family history of hypertension, and living with a smoker [Citation9,Citation23,Citation25].
Change in morning SBP from the first to the third trimester was highly influenced by the PSQI-J global score in the first trimester (r = 0.49, β = 0.58, p = .00) (), with sleep latency (r = 0.38, β = 0.43, p = .02) and sleep disturbances (r = 0.24, β = 0.33, p = .04) being the two most important components affecting this change ().
Table 4. Factors affecting changes in mean systolic blood pressure.
Table 5. Components of the PSQI-J score influencing systolic blood pressure in the first trimester to third trimester.
Discussion
We conducted this cohort study which examined the relationship between home blood pressure and sleep quality in each trimester in normal primipara women. In addition, we investigated longitudinal changes in home blood pressure and sleep quality. We demonstrated for the first time that the increase in morning SBP from the first to third trimester was associated with poor sleep quality in the first trimester, suggesting that poor sleep quality in the first trimester influenced SBP elevation. We have also found that if the quality of sleep is poor early in pregnancy, it will not change much during pregnancy and will continue to be poor. Sleep quality in the third trimester was not different in pregnant women with good sleep quality and those with poor sleep quality in the first trimester. Sleep quality of most pregnant women become worse in the third trimester because of fetal movement [Citation24]. Our finding suggests that continuous poor sleep quality from the beginning of pregnancy induces blood pressure elevation in normal primipara women.
The following points should be noted regarding our study. We evaluated blood pressure with HBPM, which is known to be much more accurate than office blood pressure for evaluation of the cardiovascular risk in patients with hypertension. Moreover, the reliability of the data was high because measured blood pressure and measurement time were recorded in the sphygmomanometer.
To the best of our knowledge, there is no concrete study of the relationship between the quality of sleep and blood pressure changes over the entire pregnancy period. A previous study on the relationship between sleep duration and office blood pressure in pregnant women reported that short or long sleep hours in the first trimester affected blood pressure in the third trimester and a sleep duration of <5 h or >10 h increased the incidence of hypertensive disorders of pregnancy and preeclampsia [Citation12]. In our study population, the relationship between sleep duration and the increase in morning SBP from the first to third trimester did not reach statistical significance (p = .06), probably because of the small number of subjects. In addition, it is said that blood pressure is affected when pre-pregnancy obesity and weight gain during pregnancy increase [Citation26]. In this study, pre-pregnancy BMI was <25 and weight gain during pregnancy was <12 kg, and these factors showed no influence on changes in blood pressure during pregnancy.
Sleep quality, including sleep duration in the first trimester, is suggested to affect blood pressure change during pregnancy. In healthy adults, it is reported that declining sleep quality is related to increased blood pressure [Citation27–29]. It has also been reported that irregular life rhythms and lifestyles are related to decreased sleep quality and an increase in blood pressure [Citation30,Citation31]. Moreover, an abnormal sleep pattern affects the normal drop in nocturnal blood pressure [Citation32]. In this study, we have proven for the first time that quality of sleep in the first trimester and blood pressure elevation during pregnancy are related. Thus, sleep quality may be a new measure to target in the prevention of pregnancy-induced hypertension.
Among adults in the general population, it has been reported that continuous insomnia is a factor that predicts high blood pressure [Citation33], and continuous positive airway pressure (CPAP) treatment can lower blood pressure in patients with sleep apnea syndrome [Citation34–36]. In addition, it is reported that CPAP treatment given to pregnant women with high blood pressure and sleeping disorders in the first trimester is effective for controlling blood pressure [Citation37,Citation38]. These findings suggest that interventions aimed at improving sleep quality may prevent blood pressure elevation during pregnancy.
The most common reasons for disturbed sleep in women in their twenties include the use of cell phones and playing games before going to bed [Citation39]. Medical personnel should assess the state of sleep and evaluate the factors that disturb sleep quality, especially in pregnant women. Giving pregnant women sleep education and helping them understand the importance of good sleep may prevent pregnancy complications. Sleep education for housemates may be also necessary. When housemates have irregular lifestyles, pregnant women often adapt to the same lifestyle. In cases with insomnia, cognitive behavioral therapy, which is known to be as effective as hypnotics, may be effective for preventing blood pressure elevation. Interventional studies aimed at improving sleep quality in pregnant women are required in the future.
Our study had some limitations. First, this study was conducted at a single clinic, and the number of subjects was small. Therefore, the results may have been affected by regional characteristics and the characteristics of the facility. To confirm our results, it is essential to conduct surveys in larger study populations in multiple facilities. Second, the recruitment period for our study was >1 year to consider the effects of season on blood pressure [Citation1]. However, the number of study subject entries in each month was not the same. Differences in temperature and life events (year-end, New Year holidays, travel, etc.) during pregnancy may affect changes in blood pressure and sleep quality. Hence, the data may be biased. In the future, investigations should be conducted with the same number of subject entries per month or season. Third, we used the PSQI-J scoring system for evaluating sleep quality. However, nap hours were not counted in the PSQI-J towards the sleep duration. It is reported that sleepiness during the day is highly prevalent in the first trimester [Citation40], and daytime dysfunction is believed to be common, although sleep duration at night is considered appropriate [Citation19]. Our data might be biased given the lack of data on nap duration. However, 71% of our study population worked in the first trimester and we assume they did not have time to nap, making this bias minimal. Fourth, PSQI-J is self-reported and therefore, a PSQI-J of ≥6, which denotes poor sleep quality, is subjective. However, not all sleeping problems are subjective. Sleeping problems are sometimes indicated by others when people experience snoring or apnea. Research in the future should investigate information from bed partners and objective data on sleep studies. Fifth, we did not evaluate the reasons for poor sleep quality. Future studies should investigate whether sleep problems in the first trimester are due to pregnancy or a pre-existing unfavorable lifestyle or environmental factors. Finally, we targeted only primipara women because the incidence of hypertensive disorders during pregnancy is different in primipara and multipara women. Moreover, the existence of older children may affect sleep quality in pregnant women. However, it is important to monitor pregnant women who are at higher risk of high blood pressure, such as those with history of hypertension and older primipara women, more closely. Thus, our data may not be generalized to all pregnant women, and the impact of sleep quality on blood pressure elevation should be examined in a wide variety of pregnant women in the future.
In summary, we established a correlation between poor sleep quality in the first trimester and changes in blood pressure during pregnancy. The increase in morning SBP from the first to third trimester is higher in pregnant women with poor sleep quality compared to pregnant women with good sleep quality. Large-scale monitoring programs should be conducted to confirm our results and to explore the reasons for poor sleep quality in pregnant women. Interventional studies are also needed to evaluate the effect of sleep quality improvement programs on the prevention of blood pressures increases during pregnancy.
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Disclosure statement
There are no conflicts of interest to declare.
Additional information
Funding
References
- Metoki H, Ohkubo T, Watanabe Y, et al. Seasonal trends of blood pressure during pregnancy in Japan: the babies and their parents' longitudinal observation in Suzuki Memorial Hospital in Intrauterine Period study. J Hypertens. 2008;26:2406–2413.
- Hermida RC, Ayala DE, Mojón A, et al. Blood pressure patterns in normal pregnancy, gestational hypertension, and preeclampsia. Hypertension. 2000;36:149–158.
- Okun ML, Schetter CD, Glynn LM. Poor sleep quality is associated with preterm birth. Sleep. 2011;34:1493–1498.
- Tranquilli AL, Giannubilo SR. Blood pressure is elevated in normotensive pregnant women with intrauterine growth restriction. Eur J Obstet Gynecol Reprod Biol. 2005;122:45–48.
- Sells CM, Feske SK. Stroke in pregnancy. Semin Neurol. 2017;37:669–678.
- Irgens HU, Reisaeter L, Irgens LM, et al. Long term mortality of mothers and fathers after pre-eclampsia: population based cohort study. BMJ. 2001;323:1213–1217.
- Lee CJ, Hsieh TT, Chiu TH, et al. Risk factors for pre-eclampsia in an Asian population. Int J Gynaecol Obstet. 2000;70:327–333.
- Bianco A, Stone J, Lynch L, et al. Pregnancy outcome at age 40 and older. Obstet Gynecol. 1996;87:917–922.
- Best Practice Guide 2015 for Care and Treatment of Hypertension in Pregnancy. Japan Society for The Study of Hypertension in Pregnancy; [Internet]. [cited 2017 Mar 7]. Available from http://minds4.jcqhc.or.jp/minds/hypertension_in_pregnancy/hypertension_in_pregnancy.pdf#search='http%3A%2F%2Fminds4.jcqhc.or.jp%2Fminds%2Fhypertension_in_pregnancy%2Fhypertension_in_pregnancy.pdf
- Government of Japan. Maternal and Child Health Act 1965 [Internet]. [cited 2017 Mar 7]. Available from http://elaws.e-gov.go.jp/search/elawsSearch/elaws_search/lsg0500/detail?lawId=340AC0000000141&openerCode=1
- Hasegawa J, Sekizawa A, Tanaka H, et al. Current status of pregnancy-related maternal mortality in Japan: a report from the Maternal Death Exploratory Committee in Japan. BMJ Open. 2016;6:e010304.
- Katsuragi S, Tanaka H, Hasegawa J, et al. Analysis of preventability of hypertensive disorder in pregnancy-related maternal death using the nationwide registration system of maternal deaths in Japan. J Matern Fetal Neonatal Med. 2018;1–7.
- Williams MA, Miller RS, Qiu C, et al. Associations of early pregnancy sleep duration with trimester-specific blood pressures and hypertensive disorders in pregnancy. Sleep. 2010;33:1363–1371.
- Abeysena C, Jayawardana P, DE A Seneviratne R. Maternal sleep deprivation is a risk factor for small for gestational age: a cohort study. Aust N Z J Obstet Gynaecol. 2009;49:382–387.
- Micheli K, Komninos I, Bagkeris E, et al. Sleep patterns in late pregnancy and risk of preterm birth and fetal growth restriction. Epidemiology. 2011;22:738–744.
- Lee KA, Gay CL. Sleep in late pregnancy predicts length of labor and type of delivery. Am J Obstet Gynecol. 2004;191:2041–2046.
- OECD. Balancing Paid Work, Unpaid Work and Leisure. Employment database 2014 [Internet]. [cited 2018 Apr 23]. Available from: http://www.oecd.org/gender/data/balancingpaidworkunpaidworkandleisure.htm
- Ministry of Health, Labour and Welfare. The National Health and Nutrition Survey in Japan 2016 [Internet]. [cited 2018 Apr 23]. Available from: https://www.mhlw.go.jp/bunya/kenkou/eiyou/dl/h28-houkoku.pdf
- Hedman C, Pohjasvaara T, Tolonen U, et al. Effects of pregnancy on mothers' sleep. Sleep Med. 2002;3:37–42.
- Zhang S, Chang C, Zhang J, et al. Correlation analysis of sleep quality and youth ischemic stroke. Behav Neurol. 2014;2014:1.
- Bansil P, Kuklina EV, Merritt RK, et al. Associations between sleep disorders, sleep duration, quality of sleep and hypertension: Results from the National Health and Nutrition Examination Survey, 2005 to 2008. J Clin Hypertens (Greenwich). 2011;13:739–743.
- Doi Y, Minowa M, Uchiyama M, et al. Psychometric assessment of subjective sleep quality using the Japanese version of the Pittsburgh Sleep Quality Index (PSQI-J) in psychiatric disordered and control subjects. Psychiatry Res. 2000;97:165–172.
- Shimamoto K, Ando K, Fujita T, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res. 2014;37:253–390.
- Suzuki S, Dennerstein L, Dennerstein L, et al. Sleeping patterns during pregnancy in Japanese women. J Psychosom Obstet Gynaecol. 1994;15:19–26.
- Makris TK, Thomopoulos C, Papadopoulos DP, et al. Association of passive smoking with masked hypertension in clinically normotensive nonsmokers. Am J Hypertens. 2009;22:853–859.
- Cedergren M. Effects of gestational weight gain and body mass index on obstetric outcome in Sweden. Int J Gynecol Obstet. 2006;93:269–274.
- Javaheri S, Storfer-Isser A, Rosen CL, et al. Sleep quality and elevated blood pressure in adolescents. Circulation. 2008;118:1034–1040.
- Carrington MJ, Trinder J. Blood pressure and heart rate during continues experimental sleep fragmentation in healthy adults. Sleep. 2008;31:1701–1712.
- Davies RJ, Belt PJ, Roberts SJ, et al. Arterial blood pressure responses to graded transient arousal from sleep in normal humans. J Appl Physiol (1985). 1993;74:1123–1130.
- Lo SH, Liau CS, Hwang JS, et al. Dynamic blood pressure changes and recovery under different work shifts in young women. Am J Hypertens. 2008;21:759–764.
- Yamasaki F, Schwartz JE, Gerber LM, et al. Impact of shift work and race/ethnicity on the diurnal rhythm of blood pressure and catecholamines. Hypertension. 1998;32:417–423.
- Alessi A, Alessi CR, Piana ER, et al. Influence of quality of sleep on the nocturnal decline in blood pressure during ambulatory blood pressure monitoring. Arq Bras Cardiol. 2002;78:218–223.
- Suka M, Yoshida K, Sugimori H. Persistent insomnia is a predictor of hypertension in Japanese male workers. J Occup Health. 2003;45:344–350.
- Giles TL, Lasserson TJ, Smith BH, et al. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;3:CD001106.
- Bazzano LA, Khan Z, Reynolds K, et al. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension. 2007;50:417–423.
- Barbe F, Duran-Cantolla J, Capote F, et al. Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;181:718–726.
- Guilleminault C, Palombini L, Poyares D, et al. Pre-eclampsia and nasal CPAP: part 1. Early intervention with nasal CPAP in pregnant women with risk-factors for pre-eclampsia: preliminary findings. Sleep Med. 2007;9:9–14.
- Poyares D, Guilleminault C, Hachul H, et al. Pre-eclampsia and nasal CPAP: part 2. Hypertension during pregnancy, chronic snoring, and early nasal CPAP intervention. Sleep Med. 2007;9:15–21.
- Ministry of Health, Labour and Welfare. National Health and Nutrition Survey in Japan. 2015;90:206.
- Shinkawa H, Shimada M, Fujita T. Sleep quality and sleepiness characteristics in first trimester expectant mothers. J Jpn Acad Midwif. 2008;22:180–188.