Abstract
Purpose: The renin-angiotensin-aldosterone system (RAAS) plays an important role in maintaining hemodynamic homeostasis. Ethnic disparities exist regarding RAAS activity due to sympathetic activity and sodium-water retention, however the implications thereof on cardiac damage is unknown. This study investigated the associations of cardiac troponin T (cTnT), N-terminal pro-brain natriuretic peptide (NTproBNP) and subclinical LVH with components of the RAAS (renin, aldosterone and aldosterone-to-renin ratio (ARR)) and copeptin in a black and white South African cohort.
Materials and methods: The study population consisted of 305 participants (black = 139, white = 166) aged 20–62 years. Serum cTnT, NTproBNP, Cornell product, components of the RAAS (active renin, aldosterone and ARR) and copeptin were determined.
Results: The black group had lower renin (p < 0.001) and higher ARR (p < 0.001), cTnT (p = 0.015) and Cornell product compared to whites (all p < 0.001). NTproBNP and copeptin were similar between the groups. After forward stepwise adjustments for multiple confounders, inverse associations of cTnT with renin (β = −0.17, p = 0.018) and aldosterone (β = −0.14, p = 0.048) as well as an inverse association between NTproBNP and aldosterone (β = −0.25, p < 0.001) were observed in the white population only. In the black group cTnT associated positively with renin (β = 0.16, p = 0.040) and copeptin (β = 0.21, p = 0.020) and inversely with ARR (β = −0.15, p = 0.047). Additionally, NTproBNP associated positively with copeptin (β = 0.18, p = 0.045). No correlations were observed between the RAAS and Cornell product in any of the groups.
Conclusions: Our findings suggest that RAAS, together with cardiac stress may function differently in cardiac damage and remodelling in the two ethnic groups; which may influence treatment in clinical practice.
Introduction
One of the major public health concerns in sub-Saharan Africa is the increasing hypertension prevalence and occurrence of cardiovascular disease (CVD) [Citation1,Citation2]. Factors linked to increased blood pressure (BP) and associated cardiac and renal damage include among others dysregulation of the renin-angiotensin-aldosterone system (RAAS), which is responsible for the control of arterial pressure, tissue perfusion and extracellular volume [Citation3,Citation4]. Indirect effects of renin have been associated with factors leading to apoptosis and necrosis of ventricular myocytes [Citation5,Citation6]. In addition to renin, aldosterone was shown to increase BP and organ damage, particularly ischemic heart disease and heart failure [Citation7,Citation8].
The RAAS is usually suppressed in black populations, possibly due to increased volume retention and sympathetic activity, resulting in exaggerated target organ damage such as left ventricular hypertrophy (LVH) and heart failure as compared to white populations [Citation9–12]. The potential role of the RAAS in cardiac injury and remodelling as indicated by cardiac troponin T (cTnT), N-terminal pro-brain natriuretic peptide (NTproBNP) and subclinical LVH may provide further insight into vascular dynamics and risk of CVD in this bi-ethnic population. Cardiac troponin T has been linked to myocardial infraction [Citation13], while NTproBNP has been associated with congestive heart failure and cardiovascular events in hypertensives with LVH [Citation14,Citation15]. Additionally, a novel stress-related neurohormone copeptin has also been associated with myocardial infarction [Citation16,Citation17]. To the best of our knowledge the potential associations between the RAAS and cardiac damage have not been investigated in a bi-ethnic population. Our aim was therefore to assess associations of cTnT, NTproBNP, subclinical LVH with components of the RAAS (renin, aldosterone and aldosterone-to-renin ratio (ARR)) and copeptin in a black and white South African cohort.
Materials and methods
Study design and population
Our sub-study is nested in the Sympathetic activity and Ambulatory Blood Pressure in Africans (SABPA) study, which was conducted from May 2008 until February 2009 [Citation18]. The study sample composed of 409 teachers working in the Kenneth Kaunda Education district in the North West Province, South Africa. The reason for this selection was to obtain a homogenous sample from a similar socio-economic class ().
The exclusion criteria for the SABPA study were: a tympanum temperature above 37.5 degrees Celsius, vaccinated or donated blood in the three months prior to commencement of the study, pregnancy and/or lactation and usage of psychotropic substances. For the purpose of our sub-study we additionally excluded anti-hypertensive medication users (n = 39) directly targeting the RAAS such as angiotensin receptor blockers (ARBs) and angiotensin converting enzyme (ACE) inhibitors and participants with a Grade I atrioventricular (AV) block (n = 65). The final study sample consisted of 305 participants, 139 black participants and 166 white participants ().
Prior to conduction of the study, written informed consent was obtained and measures and processes as well as the aims of the study were explained to each participant before signing the consent form. Assistance was made available to any participant who requested the information be conveyed in their home language. This sub-study was approved by the Ethics Committee of the North-West University (NWU-00069-17-S1) and complied with the Declaration of Helsinki criteria for Human Research (revised 2004).
Cardiovascular measurements
Each working day of the week a validated 24-h ambulatory BP monitoring (ABPM) and ECG device (CardioTENS® CE120, Meditech, Budapest, Hungary) was fitted to the non-dominant arm of four participants using an appropriate cuff size. Systolic- and diastolic BP measures as well as heart rate (HR) were obtained every 30 min during the day (08:00–22:00) and hourly during the night (22:00–06:00). Participants continued their usual daily activities and were asked to record occurrences of stress, physical activity, headache, syncope, dizziness, nausea, palpitations, hot flushes and visual disturbances on their ambulatory diary card. Hypertension was defined as ABPM ≥ 130/80 mmHg according to the European Society of Hypertension guidelines or the use of antihypertensive medication. LVH, as indicated by the Cornell product, was determined from the 10-lead ECG (NORAV Medical Ltd PC 1200, Israel, Software version 5.030) and calculated as (RaVL + SV3)*QRS duration > 244 mV.ms (LVH).
Anthropometric measurements
The height (Invicta Stadiometer, IP 1465, London, UK), weight (Precision Health Scale, A & D Company, Tokyo, Japan) and circumference of the waist and hips (Holtain non-stretchable metal flexible measuring tape) were measured in triplicate by registered level II anthropometrists in a temperature controlled private room. This was all done adhering to standardised procedures and intra- and inter-variability were less than 10%. Body mass index (BMI) was calculated and expressed as kg/m2. Total Energy Expenditure (kcal) in 24 h, taking Resting Metabolic Rate (RMR) into account was measured using the Actical® activity monitor (Mini Mitter Co., Inc.,Bend, OR; Montreal, Quebec, Canada).
Biological sampling and biochemical analyses
Participants were requested to be in a fasted state by not eating or drinking anything except water for approximately 8–10 h prior to sample collection in the mornings. Participants were in a semi-recumbent position for the resting 10-lead ECG and 30–45 min followed to adhere to posture requirements when assessing the RAAS. With the use of a sterilized winged set a registered nurse collected a fasting blood sample from the participant’s antebrachial vein branches. The samples were then prepared adhering to the standardized procedures and stored at −80 °C until analyses. With the use of sequential multiple analysers (Konelab 20i, ThermoScientific, Vantaa, Finland; and Cobas Integra 400 plus, Roche, Basel, Switzerland) total cholesterol (TC), high density lipoprotein (HDL) cholesterol, fasting glucose, high sensitivity C-reactive protein (CRP), gamma-glutamyltransferase (GGT) along with glycosylated haemoglobin (HbA1c) were analyzed. Serum cotinine was analysed with a homogeneous immunoassay (Automized Modular, Roche, Basel, Switzerland). Serum creatinine was analysed with the use of the enzymatic colorimetric test (Cobas Integra 400 plus, Roche). Glomerular filtration rate (GFR) was estimated by using the Chronic Kidney Disease (CKD) Epidemiology Collaboration (CKD-EPI) creatinine equation [Citation19]. An 8-h morning spot urine sample was collected from which creatinine, sodium and potassium were measured (Cobas Integra 400 plus, Roche, Basel, Switzerland; 3-Cat Fast Track kit, LDN, Nordhorn, Germany) and sodium-to-potassium ratio (Na+/K+) calculated. The sensitivity radio-immunometric assay was used to analyse active plasma renin in duplicate, cross-reaction with prorenin was found to be 0.4% (Renin III Generation, CIS Biointernational, codolet, France). The reagents were obtained from the mouse anti-human-active renin monoclonal antibody (IBL Lab, 38T501, Minneapolis, MN, USA). Inter- and intra-assay coefficients of variation were less than 10%. Aldosterone measurements were also conducted using the competitive radioimmunoassay (Beckman Coulter, Brea, CA). Aldosterone-to-renin ratio was calculated from plasma renin and aldosterone. Serum estradiol, cTnT and NT-proBNP were analysed with an electrochemiluminescence assay on the Cobas e411® (Roche, Basel, Switzerland) using high-sensitive methods. The inter- and intra-batch variability was 4.6 and 4.2% for cTnT and NT-proBNP, respectively. The limit of detection for cTnT was 3 pg/ml. Plasma copeptin was analysed with a sandwich immunoassay in chemiluminescence/coated tube format (ThermoFisher Scientific, B.R.A.H.M.S. Biomarkers) with the inter- and intra-batch variability being <10%.
Statistical analyses
Data analysis was performed with Statistica v13.2 (Statsoft Inc., Tulsa, USA). Single two-way general linear model interactions on main effects (ethnicity × sex) were computed for RAAS markers, independent of age, sex and waist circumference. Stratification into specific ethnic groups was motivated by the significant ethnic interactions for plasma renin [F(1, 358), 24.21, p ≤ 0.001]. All continuous variables were checked for normality by visual inspection and the Kolmogorov–Smirnov test. Non-Gaussian distributions were logarithmically transformed (including renin, aldosterone, ARR, estradiol, CRP, total energy expenditure, GGT, HBA1c, creatinine, cTnT, NTproBNP and copeptin). Independent t-tests were performed to compare groups. For categorical variables, comparisons were carried out by using of Chi-square analysis. In addition, we performed single and partial regression analyses to investigate the associations of cTnT with components of the RAAS and copeptin in the groups separately. The partial correlations were adjusted for age, sex and waist circumference. Forward stepwise multiple regressions were also performed with cTnT, NTproBNP and Cornell product as the dependent variable one at a time in all four models, while renin, aldosterone, ARR and copeptin were included in the models one at a time as main independent variables. Other independent variables included age, sex, antihypertensive medication, waist circumference, GGT, CRP, estradiol, 24-h systolic BP (SBP) and eGFR.
Results
Characteristics of the population
illustrates the characteristics of the population stratified by ethnicity. The groups were comparable regarding mean age (p = 0.15) and adiposity markers (all p ≥ 0.070). The white group had a higher total energy expenditure (p < 0.001). The black group presented with a more adverse cardiovascular profile, with higher ambulatory SBP, DBP and HR compared to whites (all p < 0.001). In addition, a larger percentage of blacks (62.6%) as opposed to whites (39.8%) were found to be hypertensive (p < 0.001). The black group also presented with lower renin (p < 0.001) and higher ARR (p < 0.001), cTnT (p = 0.015) levels and Cornell product (p < 0.001). Copeptin and NTproBNP were comparable between the groups (p ≥ 0.38) (). Urinary Na+/K+ was higher in blacks as compared to whites (p = 0.030). Gamma-glutamyl transferase, CRP and HbA1c were also higher in blacks compared to whites (all p < 0.001), while total cholesterol (p < 0.001) and HDL cholesterol (p = 0.035) were higher in whites compared to blacks.
Figure 2. Comparison of RAAS components, copeptin and cardiac injury and remodelling. *p < 0.05; †p ≤ 0.01; ‡p ≤ 0.001. Abbreviations: ARR: aldosterone-to-renin ratio; cTnT: cardiac troponin T; NTproBNP: N-terminal pro-brain natriuretic peptide.
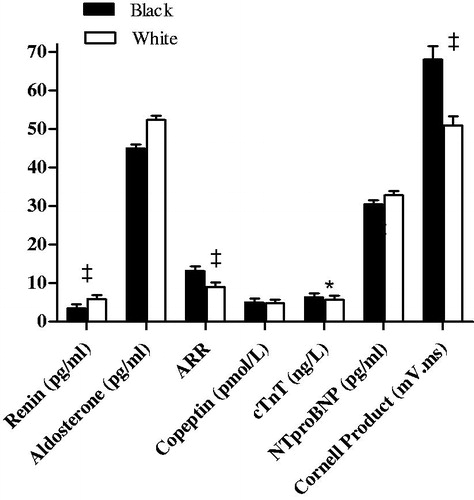
Table 1. Characteristics of the population
represents the unadjusted and adjusted correlations between cTnT and components of the RAAS (renin, aldosterone and ARR) adjusted for age, sex and waist circumference. Unadjusted correlations in the white group showed negative associations of NTproBNP with renin, aldosterone and copeptin (all p ≤ 0.008), positive association between cTnT and copeptin (p < 0.001) and a positive associations of Cornell product with renin and copeptin (both p ≤ 0.029). In the black group cTnT associated positively with copeptin (p < 0.001). After partial adjustments in the white group cTnT was found to be negatively associated with renin (p ≤ 0.001) and positively with copeptin (p = 0.047), while NTproBNP associated negatively with aldosterone (p = 0.002). In the black group both cTnT and NTproBNP associated positively with copeptin (both p ≤ 0.044) ().
Table 2. Pearson and partial correlations of cTnT, NTproBNP and Cornell product with components of the RAAS (aldosterone, renin and ARR) and copeptin
After multiple adjustments to determine independent relations of cTnT, NTproBNP and Cornell product with components of the RAAS (aldosterone, renin and ARR) and copeptin, inverse associations of cTnT with renin (β = −0.17, p = 0.018) and aldosterone (β = −0.14, p = 0.048) as well as an inverse association between NTproBNP and aldosterone (β = −0.25, p < 0.001) were observed in the white population only. In the black group cTnT associated positively with renin (β = 0.16, p = 0.040) and copeptin (β = 0.21, p = 0.020) and inversely with ARR (β = −0.15, p = 0.047). Additionally, NTproBNP associated positively with copeptin (β = 0.18, p = 0.045) ().
Table 3. Multiple regression analyses of cTnT, NTproBNP and Cornell product with components of the RAAS (aldosterone, renin and ARR) and copeptin
Discussion
Our aim was to investigate whether there is an association of cardiac injury and remodelling as indicated by cTnT, NTproBNP and subclinical LVH with components of the RAAS (renin, aldosterone and ARR) and copeptin, in a bi-ethnic South African population. A negative association existed between cTnT and RAAS (aldosterone, renin) as well as between NTproBNP and aldosterone in the white population, while in the black group cTnT was positively associated with renin and copeptin and inversely with ARR. Furthermore, NTproBNP was positively associated with copeptin. No associations were observed between Cornell product, the RAAS and copeptin in either group.
It has been established that black populations are more inclined to have a suppressed RAAS activity due to volume retention [Citation9,Citation10,Citation20]. When the risk for cardiac injury increases such as in the presence of hypertension (62.6% in the black group), renin as a RAAS trigger enzyme may decrease even further to lower BP as a homeostatic reflex against volume loading. Therefore, the positive association between cTnT and renin found in the black population indicates that increases in renin levels may contribute to cardiomyocyte injury, which was unexpected due to the low renin levels observed in the group. Possible factors driving this association could be a pre-existing vulnerable cardiovascular state due to pressure and volume-overload [Citation21] as well as higher sympathetic activation, which is well-established in the SABPA cohort [Citation11,Citation22,Citation23], and also confirmed by the positive associations of cTnT and NTproBNP with copeptin in the current sub-study only in the black group. It was previously shown that even at low active renin levels, exposure to stress resulted in a positive association between renin and total peripheral vascular resistance in a black population [Citation24]. In addition, renin was associated with an increase in ambulatory HR, while aldosterone and ARR reduced night time HR dipping in the same cohort [Citation25]. Even though the direct effects of renin on the heart are not well established, it has been suggested that a receptor for renin and its precursor protein, prorenin is present in the heart [Citation26]. Multiple studies conducted on rats found that the inhibition of the pro(renin) receptor mitigates the pathogenesis of cardiac fibrosis prompted by hypertension and remodelling [Citation26–28]. This suggests that the activation of this receptor may explain the association of renin with a marker of cardiomyocyte injury observed in the present study. The co-occurrence of the positive association of cTnT with renin and a negative association with ARR points towards a RAAS suppression mechanism to prevent further volume expansion and injury to cardiomyocytes.
It has been observed within the SABPA study that the aetiology of cardiac remodelling differs by ethnicity and potentially that of cardiac injury [Citation29]. The Jackson Heart study in African–Americans indicated that both renin and aldosterone were positively associated with incident CVD and all-cause mortality [Citation30]. In contrast, our study showed a negative association of renin and aldosterone with a marker of cardiomyocyte injury in the white group whereas a positive association between renin and cTnT was observed only in the black population, the latter concurring with findings in African-Americans. The negative association of cTnT and NTproBNP with renin and aldosterone in the whites may be suggestive of a negative feedback mechanism for BP control [Citation31]. It underscores our findings of higher renin and aldosterone levels along with lower HR and BP in the white population as compared to the black population. In addition to the possible role of renin in cardiac injury, aldosterone has been implicated in reperfusion injury due to its activation of genomic and non-genomic pathways in the cardiovascular system [Citation32,Citation33]. Of note is that despite the negative association of the RAAS with cardiac damage in the white group, copeptin was associated positively with possible cardiac stress as indicated by NTproBNP. Copeptin is regarded as a surrogate vasopressin marker and therefore increased plasma volume may be the mechanism underlying the observed positive association [Citation17], with subsequent inhibition of the RAAS [Citation34] as indicated by a negative association between NTproBNP and aldosterone in our white group.
The lack of associations between a marker of subclinical LVH, RAAS components and copeptin, may suggest that the BP and volume regulatory mechanisms may still be sufficient in protecting the cardiac muscle against pressure-overload that can result in significant cardiac remodelling in the form of LVH. Left ventricular hypertrophy is usually regarded as a maladaptive response to chronic pressure overload which may stimulate left ventricular myocardial growth and neuro-humoral stimuli which then initiate and maintain a hypertrophic response [Citation35]. On the other hand, cTnT is released as a result of disruption of the protein complexes as cardiomyocytes are damaged due to ischemia [Citation36], potentially the case in our black group.
These results should be interpreted within the context of the strengths and limitations of the study. Participants with antihypertensive medication use, such as beta blockers, diuretics and calcium channel blockers were not excluded and we therefore cannot exclude residual confounding. Due to the cross-sectional design of the study, causality cannot be inferred. However, the study was conducted under well-controlled experimental conditions and included a cohort of urban-dwelling black and white Africans matched for socio-economic status, although we could not control cultural differences. Even though the study population cannot be regarded as representative of the general South African population, it provides some much-needed knowledge regarding the role of the RAAS in cardiovascular health.
In conclusion, we found an adverse association of makers of cardiac injury with renin and copeptin in the black population, while the white group seemed to present with a negative feedback loop between copeptin, the RAAS and cardiac injury and remodelling. A pre-existing vulnerable cardiovascular state in black participants may predispose the group to cardiomyocyte injury due to RAAS dysregulation. Interactions with other blood pressure-regulating pathways may influence treatment in clinical practice.
Acknowledgements
The authors thank the participants of the SABPA study for their voluntary participation and staff members for data collection and analyses.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Tibazarwa KB, Damasceno AA. Hypertension in developing countries. Can J Cardiol. 2014;30:527–533.
- Lloyd-Sherlock P, Beard J, Minicuci N, et al. Hypertension among older adults in low-and middle-income countries: prevalence, awareness and control. Int J Epidemiol. 2014;43:116–128.
- Ferrario CM, Strawn WB. Role of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular disease. Am J Cardiol. 2006;98:121–128.
- Ma T, Kam K, Yan B, et al. Renin-angiotensin-aldosterone system blockade for cardiovascular diseases: current status. Br J Pharmacol. 2010;160:1273–1292.
- Sato Y, Fujiwara H, Takatsu Y. Biochemical markers in heart failure. J Cardiol. 2012;59:1–7.
- Azzam ZS, Kinaneh S, Bahouth F, et al. Involvement of cytokines in the pathogenesis of salt and water imbalance in congestive heart failure. Front Immunol. 2017;8:716.
- Ferreira J, Santos M, Almeida S, et al. High-dose spironolactone changes renin and aldosterone levels in acutely decompensated heart failure. Cor Vasa. 2014;56:e463–e470.
- du Cailar G, Fesler P, Ribstein J, et al. Dietary sodium, aldosterone, and left ventricular mass changes during long-term inhibition of the renin-angiotensin system. Hypertension. 2010;56:865–870.
- Lindhorst J, Alexander N, Blignaut J, et al. Differences in hypertension between blacks and whites: an overview. Cardiovasc J Afr. 2007;18:241–247.
- Schutte AE, Botha S, Fourie CMT, et al. Recent advances in understanding hypertension development in sub-Saharan Africa. J Hum Hypertens. 2017;31:491–500.
- Myburg CE, Malan L, Wentzel A, et al. Coping and cardiac troponin T-A risk for hypertension and sub-clinical ECG left ventricular hypertrophy: the SABPA study. Heart Lung Circ. 2019;28:908–916.
- Sliwa K, Wilkinson D, Hansen C, et al. Spectrum of heart disease and risk factors in a black urban population in South Africa (the Heart of Soweto Study): a cohort study. Lancet. 2008;371:915–922.
- Braunwald E, Antmann EM, Beasley JW, et al. ACC/AHA guidelines for the management of patients with unstable angina and non ST elevation myocardial infarction: executive summary and recommendations. A report of the American College of Cardiology/American Heart Association task force on practice guidelines (committee on the management of unstable angina). Circulation. 2000;102:1193–1209.
- Ozturk TC, Unluer E, Denizbasi A, et al. Can NT-proBNP be used as a criterion for heart failure hospitalization in emergency room? J Res Med Sci. 2011;16:1564–1571.
- Takeda T, Kohno M. Brain natriuretic peptide in hypertension. Hypertens Res. 1995;18:259–266.
- Khan SQ, Dhillon OS, O’Brien RJ, et al. C-terminal proVasopressin (copeptin) as a novel and prognostic marker in acute myocardial infarction-The Leicester Acute Myocardial Infarction Peptide (LAMP) Study. Circulation. 2007;115:2103–2110.
- Katan M, Morgenthaler N, Widmer I, et al. Copeptin, a stable peptide derived from the vasopressin precursor, correlates with the individual stress level. Neuro Endocrinol Lett. 2008;29:341–346.
- Malan L, Hamer M, Frasure-Smith N, et al. Cohort profile: Sympathetic activity and Ambulatory Blood Pressure in Africans (SABPA) prospective cohort study. Int J Epidemiol. 2015; 44:1814–1822.
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612.
- Seedat Y. Hypertension in black South Africans. J Hum Hypertens. 1999;13:96–103.
- Tomaschitz A, Pilz S, Ritz E, et al. Associations of plasma renin with 10-year cardiovascular mortality, sudden cardiac death, and death due to heart failure. Eur Heart J. 2011;32:2642–2649.
- Malan L, Hamer M, von KR, et al. Chronic defensiveness and neuroendocrine dysfunction reflect a novel cardiac troponin T cut point: the SABPA study. Psychoneuroendocrinology. 2017;85:20–27.
- Hamer M, Malan L, Schutte AE, et al. Plasma renin responses to mental stress and carotid intima-media thickness in black Africans: the SABPA study. J Hum Hypertens. 2011;25:437–443.
- Gafane LF, Schutte R, Van Rooyen JM, et al. Plasma renin and cardiovascular responses to the cold pressor test differ in black and white populations: The SABPA study. J Hum Hypertens. 2016;30:346–351.
- Gafane-Matemane LF, Van Rooyen JM, Schutte R, et al. Aldosterone and renin in relation to surrogate measures of sympathetic activity. Cardiovasc J Afr. 2019;30:34–40.
- Nguyen G, Delarue F, Burcklé C, et al. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109:1417–1427.
- Ellmers LJ, Rademaker MT, Charles CJ, et al. (Pro)renin receptor blockade ameliorates cardiac injury and remodeling and improves function after myocardial infarction. J Card Fail. 2016;22:64–72.
- Ichihara A, Kaneshiro Y, Takemitsu T, et al. Nonproteolytic activation of prorenin contributes to development of cardiac fibrosis in genetic hypertension. Hypertension. 2006;47:894–900.
- Van Vuren EJ, Malan L, von KR, et al. Hyperpulsatile pressure, systemic inflammation and cardiac stress are associated with cardiac wall remodelling in an African male cohort: the SABPA study. Hypertens Res. 2016;39:648–653.
- Joseph JJ, Echouffo-Tcheugui JB, Kalyani RR, et al. Aldosterone, renin, cardiovascular events, and all-cause mortality among African Americans: the Jackson Heart Study. JACC Heart Fail. 2017;5:642–651.
- Guyton AC, Coleman TG, Cowley AW, et al. Arterial pressure regulation: overriding dominance of the kidneys in long-term regulation and in hypertension. Am J Med. 1972;52:584–594.
- Ashton AW, Le TY, Gomez-Sanchez CE, et al. Role of nongenomic signaling pathways activated by aldosterone during cardiac reperfusion injury. Mol Endocrinol. 2015;29:1144–1155.
- Xiao TT, Wang YY, Zhang Y, et al. Similar to spironolactone, oxymatrine is protective in aldosterone-induced cardiomyocyte injury via inhibition of calpain and apoptosis-inducing factor signaling. PLOS One. 2014;9:e88856.
- deFilippi CR, Christenson RH. B-Type natriuretic peptide (BNP)/NT-proBNP and renal function: is the controversy over? Clin Chem. 2009;55:1347–1353.
- Katholi RE, Couri DM. Left ventricular hypertrophy: major risk factor in patients with hypertension: update and practical clinical applications. Int J Hypertens. 2011;2011:1.
- Sharma S, Jackson PG, Makan J. Cardiac troponins. J Clin Pathol. 2004;57:1025–1026.