Abstract
Purpose: In the course of hypertension, left ventricular hypertrophy and diastolic dysfunction develop very often and may progress toward heart failure. The aim of the study was to analyze the relationship between abnormalities of retinal microcirculation and cardiac damage defined as left ventricular hypertrophy and/or diastolic dysfunction.
Materials and methods: The study comprised 88 patients with essential hypertension. The group was divided into two subgroups: hypertensives without cardiac damage (n = 55) and with cardiac damage (n = 33). Control group comprised 32 normotensive subjects. Scanning laser Doppler flowmetry was used to evaluate retinal microcirculation. Echocardiography was used to assess cardiac damage.
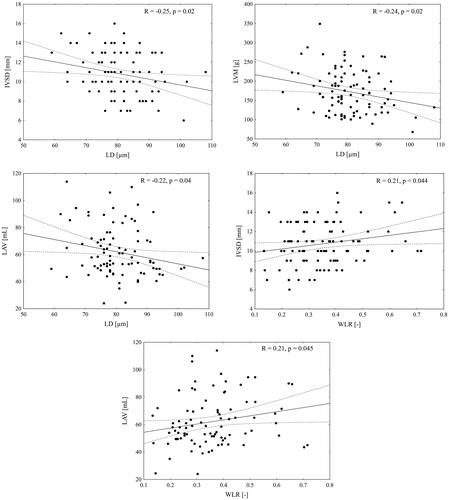
Results: Lumen diameter of retinal arterioles was significantly smaller in patients with cardiac damage vs. controls (77 vs. 84 µm, p = 0.02). Additionally, there was an evident trend with respect to lumen diameter (LD) across all three studied subgroups; i.e.: the smallest dimeters were present in cardiac damage patients, moderate size in hypertensives’ without cardiac damage, and the largest diameters in healthy controls (pfor trend < 0.01). Lumen diameter was inversely correlated with cardiac intraventricular septum diameter (R = –0.25, p = 0.02), left ventricular mass (R = –0.24, p = 0.02), and left atrial volume (R = –0.22, p = 0.04). Wall to lumen ratio was associated with intraventricular septum diameter (R = 0.21, p = 0.044) and left atrial volume (R = 0.21, p = 0.045). In multivariable regression analysis, lumen diameter was independently associated with intraventricular septum diameter (β = –0.05, p = 0.03), left ventricular mass (β = –1.15, p = 0.04), and left atrial volume (β = –0.42, p = 0.047); wall to lumen ratio was independently associated with intraventricular septum diameter (β = 3.67, p = 0.02) and left atrial volume (β = 30.0, p = 0.04).
Conclusions: In conclusion, retinal arterioles lumen diameter and wall to lumen ratio were independent biomarkers of cardiac damage. Retinal examination performed by means of scanning laser Doppler flowmetry might be a valuable tool to improve cardiovascular risk stratification of hypertensive patients.
Introduction
Heart failure has become a global pandemic, since it affects at least 26 million people worldwide and its prevalence is still rising with population ageing [Citation1]. In hypertensive subjects the lifetime risk for developing heart failure is twofold higher in men and threefold higher in women comparing to normotensive individuals [Citation2]. In the course of hypertension, left ventricular hypertrophy and diastolic dysfunction develop very often as a manifestation of target organ damage [Citation3] and may evolve toward heart failure [Citation4,Citation5]. In the recent guidelines for heart failure management [Citation6], the presence of left ventricular hypertrophy or diastolic dysfunction are the main echocardiographic criteria required to establish diagnosis of HF with preserved or mid-range ejection fraction. Taking into consideration that myocardial remodelling starts before the onset of full-blown heart failure [Citation7], we should place a special emphasis on looking for abnormalities preceding this process. Indeed, microvascular changes were found to be a pathogenic factor contributing to different cardiac diseases [Citation8–12]. Of clinical importance, microvascular impairments were related to increased cardiovascular mortality in short [Citation13,Citation14] and long term follow-up [Citation15]. For these reasons, microvascular abnormalities appear to be prognostically relevant biomarkers that might be useful in identifying patients at a high risk of cardiovascular events. In majority of previous investigations, techniques evaluating microcirculation were characterized by invasive nature requiring biopsy of gluteal region or poor reproducibility [Citation16–18], therefore theirs clinical utility in everyday practice was controversial. In contrast to these investigations, our study was based on noninvasive and in vivo evaluation of microcirculation by means of scanning laser Doppler flowmetry (SLDF) [Citation19]. This method was proved to provide similar information about microvascular structure as validated and prognostically relevant micromyography of subcutaneous small arteries [Citation20], suggesting that abnormalities of microvessels can be simultaneously present in different microvascular districts. Thus, we hypothesized that remodelling of retinal microvessels may reflect cardiac microvascular disease and serve as a useful biomarker to assess changes, which occur in the heart in course of hypertension. The novelty of the study is that we aimed by means of completely noninvasive and in vivo approach – SLDF – to establish (1) the association between structural and functional abnormalities of retinal microcirculation and cardiac damage defined as left ventricular hypertrophy and/or diastolic dysfunction, and (2) to explore whether assessment of microvascular changes could add information in the prediction of cardiac damage.
Methods
Study population
Patients were recruited from the outpatient clinic of the Department of Hypertension and Diabetology, Faculty of Medicine, Medical University of Gdansk, Gdansk, Poland between October 2015 and April 2016.
The only screening inclusion criteria were (1) primary hypertension treated for at least one year and (2) age of 40–70 years old. Exclusion criteria were as follows: (1) any form of secondary hypertension (based on medical records); (2) cardiovascular disease including heart failure, coronary artery disease, arrhythmia, moderate or severe valvular heart disease; (3) history of stroke or transient ischemic attack; (4) chronic kidney disease with estimated glomerular filtration rate (eGFR) <60 mml/min/1.73 m2; (5) diabetes mellitus; (6) eye disease (glaucoma, cataract); (7) mental disorders including depression, and (8) pregnancy. From 867 consecutive records of hypertensive database, 120 consecutive patients were initially selected on the basis of the study criteria and invited for further examinations. Medical interview, physical examination, blood pressure measurement, echocardiography, electrocardiography, and scanning laser Doppler flowmetry were performed during first meeting. Laboratory tests were done on the next day. All patients were examined during antihypertensive therapy. Finally, 88 subjects – fully meeting the study criteria – were enrolled and analyzed.
Patients were divided into two subgroups:
Hypertensive patients with cardiac damage defined as the presence of diastolic dysfunction and/or left ventricle hypertrophy in echocardiography (n = 33)
Hypertensive patients without cardiac damage (n = 55).
Additionally, we included 32 healthy participants matched in terms of age and gender as a control group.
The study protocol was approved by the Ethics Committee of Medical University of Gdansk, Poland and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Written informed consent of each participant was obtained before the study inclusion.
Retinal arteriolar remodelling assessment
Retinal arteriolar structure and function were evaluated by means of scanner laser Doppler flowmetry with 670 nm laser beam (SLDF, Heidelberg Retina Flowmetry, Heidelberg Engineering GmbH Germany). The principles of SLDF have been described in detail [Citation21,Citation22]. The examination did not require any pharmacological pupil dilatation, thus we avoided artificial influence on vascular tone and flow. Measurements were performed in a dark room, in a sitting position, after 15 min of rest. Confocal technique of device allowed the evaluation of the arterioles only from superficial retinal layer. All measurements were taken in juxtapapillary area of the right eye, 2–3 mm temporally superior to the optic nerve [Citation23]. The retinal sample of 2.56 × 0.64 × 0.3 mm at a resolution of 256 points × 64 lines × 128 lines was scanned within 2 seconds. All images were analyzed offline by Automatic Full Field Perfusion Image Analysis. Three images of each patient were taken for the analysis. Outer diameter (OD) was measured in reflection images, and lumen diameter (LD) was measured in perfusion images. SLDF allows precise and reliable assessment of both structure and function of microvessels; the documented test–retest and inter- and intraobserver tests coefficients of variation were less than 10% for nearly all measured parameters [Citation19].
Measurement of structural parameters
Wall to lumen ratio (WLR) was calculated using the formula: WLR = OD – LD/LD.
Wall thickness (WT) was calculated using the formula: WT = OD – LD/2.
Wall cross sectional area (WCSA) was calculated using the formula: WCSA = π/4 × (OD2 – LD2).
Measurements of retinal capillary flow (RCF)
The perfusion images were derived from the Doppler frequency shift of the reflected light by moved erythrocytes in the blood flow. Perfusion map was obtained from images by means of Automatic Full Field Analysis of Perfusion. The mean capillary flow was calculated automatically in the measured area and was expressed in arbitrary units [AU].
Echocardiography
Echocardiography was performed using a Vivid 7 Pro device with a 2.5-MHz phased-array probe (GE Vingmed Ultrasound AS, Horten, Norway). The detailed echocardiographic examination is available in the online supplement materials.
Blood pressure measurement
Brachial blood pressure (BP) was obtained by the validated oscillometric device on the non-dominant arm, in supine position, after 15 minutes of rest, using an appropriate cuff size for the arm circumference [Citation24]. An average of three measurements at intervals of 5 minutes was taken into analysis.
Statistical analysis
Quantitative data are presented as means with standard deviations (SDs), or medians with interquartile ranges (IQRs). Categorical variables are described as numbers and percentages. The assumption of normal distribution was tested using Shapiro–Wilk and Anderson–Darling tests. Multiple comparisons were performed using one-way analysis of variance (ANOVA) with Tukey’s HSD post hoc test or Kruskal–Wallis test with Dunn’s post hoc test for the quantitative variables, whereas chi-square or Fisher’s exact tests were applied to qualitative data. The linear trends between groups were evaluated using ANOVA with polynomial contrast or Jonckheere–Terpstra trend test. The relationship between the two continuous variables was investigated using either Pearson product–moment correlation or Spearman’s rank correlation. Multivariate models were based on linear regression. Results from linear regressions were expressed as beta coefficient with 95% confidence intervals (CIs). The goodness of fit in multiple regression was assessed using Akaike information criterion (AIC) and Bayesian information criterion (BIC). Variance inflation factor (VIF) was calculated to estimate the possibility of multicollinearity among predictors.
The two-tailed tests were carried out at a significance level of p < 0.05. All statistical analyses were performed with R package version 3.2.3 and Statistica 13.1.336.0 (StatSoft Inc., Tulsa OK Oklahoma, USA).
Results
Baseline characteristics
Mean age of the whole study group was 54.3 ± 8.3 years and 45% (n = 54) were women. Baseline characteristics comparing patients with and without cardiac damage are presented in . Patients with cardiac damage had higher body mass index (BMI), eGFR, brachial systolic (bSBP) and mean blood pressure (bMBP) comparing to subjects without cardiac damage. Age, sex, WHR and other laboratory tests results did not differ between these subgroups. In terms of pharmacological treatment, there were no differences between subgroups of patients with hypertension except for CCBs and statins use; both were more frequently administered in patients with cardiac damage ().
Table 1. Baseline characteristics of the study group.
Cardiac and microvascular parameters
In of the Supplemental material, we compared parameters of cardiac and microvascular structure and function between the groups. In terms of cardiac parameters IVSD, PWD, LVM, LVMI, LAV, LAVI, IVRT, and E/E’ were significantly higher in hypertensive patients without cardiac damage versus normotensive individuals. E’ivs, A’lw, and E’mean were significantly lower in patients without cardiac damage versus normotensive individuals. In terms of retinal parameters, lumen diameter was significantly smaller in hypertensive patients with cardiac damage than in normotensive individuals.
Table 3. Multivariate regression analysis evaluating the association of retinal parameters with cardiac damage indices
In , we depicted that lumen diameter decreased from normotensive subjects to hypertensive patients without and then with cardiac damage (ptrend = 0.006) Moreover, lumen diameter was inversely correlated with IVSD, LVM, and LAV (). WLR was associated with IVSD and LAV, however, we have not found any significant correlation with LVM (data not shown). Taking into consideration potentially confounding factors like age, sex and mean BP we included them in multivariable analysis which evaluated the impact of retinal parameters on cardiac remodelling (). We have revealed that lumen diameter was independently associated with IVSD, LVM, and LAV. Moreover, WLR was revealed as an independent predictor of LAV and IVSD in performed analysis. We also evaluated the impact of LD and WLR on E′ mean and E/E′, however, we have not found any significant association (data not shown).
Figure 1. Lumen diameter of retinal arterioles in 3 subgroups: 0 – healthy subjects, 1 – hypertensive patients without cardiac damage, 3 – hypertensive patients with cardiac damage. The narrowest lumen corresponds to the presence of cardiac damage (Jonckheere-Terpstra trend test).
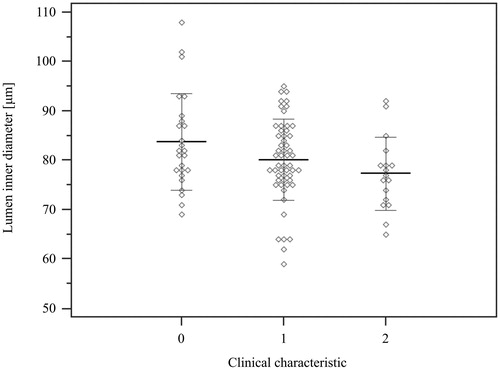
Figure 2. Correlation analysis between cardiac and retinal parameters. Structural microvascular abnormalities are associated with indices of cardiac damage: lumen diameter (LD) is associated with intraventricular septum (IVSD), left ventricular mass (LVM) and left atrial volume (LAV), whereas wall to lumen ratio (WLR) is associated with IVSD and LAV (Spearman’s rank correlation analysis).
Discussion
The novel findings of our study are the following:
Retinal examination performed by means of SLDF has a significant value in detecting structural vascular abnormalities parallel to cardiac damage.
Lumen narrowing of retinal arterioles was independently related to intraventricular septum thickness, left ventricular mass and left atrial volume.
Wall lumen ratio was independently associated with intraventricular septum and left atrial volume.
The importance of retinal examination in the context of cardiovascular diseases has been proved in many previous investigations. The first description of hypertensive retinopathy was already made in the nineteenth century by a Scottish physician Robert Marcus Gunn [Citation17]. Afterwards, in 1939 Keith et al. have provided a classification of hypertensive retinopathy and examined the impact of retinal changes on survival of hypertensive patients [Citation17]. Starting with these two pioneering studies through the next investigations of mainly two last decades, retinal vasculature has been revealed as a mirror of cardiovascular health allowing noninvasive and in vivo examination.
The present study has provided strong evidence that retinal microvascular remodelling was associated with cardiac organ damage. Although it is not the first investigation evaluating this relationship, it is however the first one which is based on such a precise, reliable and noninvasive approach to evaluate microcirculation as SLDF. Lumen narrowing of retinal arterioles was inversely correlated with IVSD and LVM, suggesting that it plays an important role in LVH – considered as the most relevant prognostic index of target organ damage in hypertensive patients [Citation25]. Moreover, we have found that lumen narrowing was independently and inversely associated with LA enlargement that reflects chronically elevated filling pressures and indicates for diastolic dysfunction [Citation26]. Of clinical importance, LVH and LA enlargement are relevant predictors of cardiovascular morbidity and mortality [Citation17,Citation27,Citation28]. Importantly, this association has remained significant after adjustment for age, sex and mean BP.
Proper understanding of hypertension attributable target organ damage requires integrative approach to the coupling of vascular tree and the heart [Citation29]. Microvasculature, macrovasculature and the heart are three deeply interrelated districts that altogether determine hemodynamics of circulatory system [Citation30,Citation31]. Small resistance arteries are the main determinant of total peripheral resistance. The reduced lumen diameter, increased wall to lumen ratio accompanied by structural and functional rarefaction lead to an increase of mean BP [Citation32]. Augmented mean BP contributes to the loading of stiff components in the arterial wall and subsequently enhances large artery stiffness [Citation32,Citation33], which in turn decreases the ability of the arteries to accommodate the volume of blood ejected from the left ventricule [Citation30]. With arterial stiffening, the reflected wave moves more rapidly along the arterial tree. Early reflected wave, arriving in late systole, superimposes on the forward wave and augments the systolic pressure further. As a consequence, increased SBP and PP lead to greater LV afterload, hypertrophy of cardiomyocytes and increased myocardial oxygen consumption [Citation32]. In turn, within myocardial interstitium, collagen deposition promotes abnormal fibrosis, restricting myocardial elasticity [Citation34–36]. Myocardial hypertrophy and fibrosis lead to impaired relaxation and augmented LV filling pressures – the antecedents of further remodelling – atrial and ventricular enlargement and diastolic dysfunction [Citation37,Citation38]. This mechanism – focused on the cascade of pathological changes going from microcirculation through macrocirculation to the heart – demonstrates an indirect link between retinal vascular diameters and cardiac remodelling and only in part elucidates our results.
The second explanation is that microvascular abnormalities of the retina occur in parallel and may be translated to microvascular changes in myocardium. Previous studies have delivered a large body of evidence that coronary and retinal circulations might share the same pathogenic process [Citation39,Citation40]. In response to elevated blood pressures, myogenic tone mediates in resistance vasculature structural changes such as lumen narrowing, wall thickening and then vasocontriction. Hence, myogenic tone plays a key role in blood flow autoregulation and stabilization of capillary pressure protecting from hypertensive injury of target organs [Citation32,Citation41,Citation42]. However, in the course of chronically elevated blood pressures prolonged myogenic vasoconstriction may lead to impairment of vasodilator reserve and capillary rarefaction that both promote tissue hypoperfusion and finally impair organ function [Citation32]. According to the studies showing that alterations in small resistance arteries may be present at the same time in different vascular districts [Citation43,Citation44], structural changes that occur in retinal microvasculature might be automatically expected in microvessels of myocardium, which is exceptionally susceptible to deleterious effect of ischemia.
Adding indirect and direct impact of microvascular remodelling and increased total vascular resistance on the myocardial function, we can estimate the following adaptive changes in the heart: hypertrophy of cardiomyocytes in response to pressure overload, atrial and ventricular enlargement as a consequence of augmented filling pressures, ischemia as the result of microvascular vasoconstriction, reduced vasodilatory reserve and decreased density of arterioles in hypertrophied myocardium. Altogether, finally, lead to diastolic and systolic dysfunction and subsequently to heart failure. These associations were observed in our study in inverse and independent relationship between lumen diameter and IVSD, LVM and LAV. Since this close relation between morphology of retinal arterioles and geometric patterns of the heart is observed, our findings may have significant clinical implications.
Importantly, our results were also consistent with other previous research. The Multi Ethnic Study of Atherosclerosis has revealed by means of retinal photographs an association between retinal arteriolar narrowing and left ventricular concentric remodelling in population of 4593 subjects [Citation45]. Additionally, in a cross sectional study of 1439 African- American participants (The Atherosclerotic Risk in Communities Study – ARIC Study), LVM was inversely associated with a significant trend across the quartiles of retinal arterioles diameter, independently of age, sex, cardiovascular risk factors and hypertension-related risk factors [Citation46].
The investigation of Strain et al. has shown that microcirculatory dysfunction was also related to left atrial enlargement independently of cardiovascular risk factors including blood pressure [Citation27]. In the study of Wang et al. the narrower retinal arterioles were related to lower hyperemic myocardial blood flow and lower perfusion reserve in asymptomatic patients with no coronary calcification indicating that retinal changes may serve as a marker of coronary microvascular disease [Citation39]. Moreover, Wong et al., basing also on ARIC study population, have revealed that retinopathy was an independent predictor of congestive heart failure, even in subjects without preexisting coronary heart disease, diabetes or hypertension [Citation47]. All the above associations in combination with the fact that microvascular remodelling represents probably the earliest form of target organ damage [Citation48] allow us to consider abnormalities in retinal microvasculature as a valuable screening tool and a powerful predictor of cardiovascular risk [Citation49]. Furthermore, identification of asymptomatic subjects due to the presence of biomarkers localized in retinal microcirculation, may also provide new insight into treatment management and improve CV outcomes. Importantly, the relevant advantage of retinal vasculature above the other microvascular beds is that it provides a unique opportunity to study microvascular changes outside invasive procedure or time consuming examination.
In our study we tried to detect whether ongoing pharmacological treatment might had an impact on the SLDF-derived indices, yet the study was underpowered for such multivariate approach. Based on crude data, we found no differences in most of hypotensive agents use including ACE-I/ARB between the groups with and without cardiac damage. However, the use of calcium channel blockers was more frequent in patients with cardiac functional and structural changes.
Some limitations of the current study need to be considered. First, because of the cross sectional character of the study, it was impossible to determine antecedent-consequent associations between changes of microcirculation and the heart. Thus, future investigations should provide a prospective evaluation of this relationship. Second, due to very restrictive enrollment criteria to the study, the patients group was relatively small, which limited the possibility of including more predictors in multivariate analysis. On the other hand, we obtained a very well phenotyped group that allowed statistical analysis with significantly reduced potential confounding impact of cardiovascular diseases on the results. Third, because all hypertensive patients were subjected to antihypertensive treatment that potentially could reduce and even completely reverse cardiac and vascular remodelling, we suppose that the relationship between microcirculation and the heart could be much stronger before pharmacotherapy and the contrast between the hypertensive and normotensive groups might be more pronounced. Finally, although we aimed to select a study group free of any cardiovascular disease, we could not eliminate the presence of hyperlipidemia that was very frequent in both hypertensive and normotensive groups.
Conclusions
In conclusion, the examination of retinal circulation appears to be an excellent tool to evaluate target organ damage and may provide a window to the heart. For the first time, we have revealed that lumen narrowing and increased WLR of retinal arterioles – measured by means of noninvasive and in vivo approach – were independently associated with IVS thickness/LVM and LAV, suggesting that microvascular changes occur in parallel to cardiac remodelling. These novel findings might improve risk stratification and treatment management of hypertensive patients. Further research, however, are needed to establish whether morphologic changes of retinal vasculature may precede the development of cardiac remodelling and heart failure.
Supplemental_Materials_-_Echo_and_Table_2.docx
Download MS Word (25.1 KB)Acknowledgments
We gratefully acknowledge for excellent technical assistance of Gabriela Gierszewska, Jadwiga Ustimowicz and Wiesława Kucharska.
Disclosure statement
KN received honoraria or consultation fees from Servier, Krka, Berlin-Chemie/Menarini, Egis, Sandoz, Idorsia, Polpharma, Gedeon Richter, and ResMed. JW received honoraria or consultation fees from Servier, Krka, Berlin-Chemie/Menarini, Sandoz, and ResMed.
Additional information
Funding
References
- Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev. 2017;3(1):7–11.
- Levy D, Larson MG, Vasan RS, et al. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–1562.
- Levy D, Garrison RJ, Savage DD, et al. Prognostic implications of echocardiographically-determined left ventricular mass in the Framingham Study. N Engl J Med. 1990;322:1561–1566.
- Gradman AH, Alfayoumi F. From left ventricular hypertrophy to congestive heart failure: management of hypertensive heart disease. Prog Cardiovasc Dis. 2006;48:326–341.
- Kuznetsova T, Herbots L, Jin Y, et al. Systolic and diastolic left ventricular dysfunction: from risk factors to overt heart failure. Expert Rev Cardiovasc Ther. 2010; 8:251–258.
- Ponikowski P, Voors AA, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Eur Heart J. 2016;14:372129–372200.
- Kuznetsova T, Herbots L, López B, et al. Prevalence of left ventricular diastolic dysfunction in a general population. Circ Heart Fail. 2009;2:105–112.
- Dahlöf B, Stenkula S, Hansson L. Hypertensive retinal vascular changes: relationship to left ventricular hypertrophy and arteriolar changes before and after treatment. Blood Press. 1992;1:35–44.
- Marcus ML, Chilian WM, Kanatsuka H, et al. Understanding the coronary circulation through studies at the microvascular level. Circulation. 1990;82:1–7.
- Hellmann M, Roustit M, Cracowski JC. Skin microvascular endothelial function as a biomarker in cardiovascular diseases? Pharmacol Rep. 2015;67:803–810.
- Liu PP, Mak S, Stewart DJ. Potential role of the microvasculature in progression of heart failure. Am J Cardiol. 1999;84:23L–26L.
- Liew G, Mitchell P, Rochtchina E, et al. Fractal analysis of retinal microvasculature and coronary heart disease mortality. Eur Heart J. 2011;32:422–429.
- Wang JJ, Liew G, Klein R, et al. Retinal vessel diameter and cardiovascular mortality: pooled data analysis from two older populations. Eur Heart J. 2007;28:1984–1992.
- Witt N, Wong TY, Hughes AD, et al. Abnormalities of retinal microvascular structure and risk of mortality from ischemic heart disease and stroke. Hypertension. 2006;47:975–981.
- Mutlu U, Ikram MK, Wolters FJ, et al. Retinal microvasculature is associated with long-term survival in the general adult Dutch population. Hypertension. 2016;67:281–287.
- Favero G, Paini A, De Ciuceis C, et al. Changes in extracellular matrix in subcutaneous small resistance arteries of patients with essential hypertension. Blood Press. 2018;27:231–239.
- Cuspidi C, Negri F, Giudici V, et al. Retinal changes and cardiac remodelling in systemic hypertension. Ther Adv Cardiovasc Dis. 2009;3:205–214.
- Muiesan ML, Rizzoni D, Salvetti M, et al. Structural changes in small resistance arteries and left ventricular geometry in patients with primary and secondary hypertension. J Hypertens. 2002;20:1439–1444.
- Harazny JM, Raff U, Welzenbach J, et al. New software analyses increase the reliability of measurements of retinal arterioles morphology by scanning laser Doppler flowmetry in humans. J Hypertens. 2011;29:777–782.
- Rizzoni D, Porteri E, Duse S, et al. Relationship between media-to-lumen ratio of subcutaneous small arteries and wall-to-lumen ratio of retinal arterioles evaluated noninvasively by scanning laser Doppler flowmetry. J Hypertens. 2012;30:1169–1175.
- Michelson G, Schmauss B, Langhans MJ, et al. Principle, validity, and reliability of scanning laser Doppler flowmetry. J Glaucoma. 1996;5:99–105.
- Michelson G, Welzenbach J, Pal I, et al. Automatic full field analysis of perfusion images gained by scanning laser Doppler flowmetry. Br J Ophthalmol. 1998;82:1294–1300.
- Kannenkeril D, Harazny JM, Bosch A, et al. Retinal vascular resistance in arterial hypertension. Blood Press. 2018;27:82–87.
- Williams B, Mancia G, Spiering W, et al. 2018 Practice guidelines for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. Blood Press. 2018;27:314–340.
- Saitoh M, Matsuo K, Nomoto S, et al. Relationship between left ventricular hypertrophy and renal and retinal damage in untreated patients with essential hypertension. Intern Med. 1998;37:576–580.
- Tsang TS, Barnes ME, Gersh BJ, et al. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol. 2002;90:1284–1289.
- Strain WD, Chaturvedi N, Hughes A, et al. Associations between cardiac target organ damage and microvascular dysfunction: the role of blood pressure. J Hypertens. 2010;28:952–958.
- Vakili B, Okin P, Devereux RB. Prognostic implications of left ventricular hypertrophy. Am Heart J. 2001;141:334–341.
- Struijker Boudier HA, Cohuet GM, Baumann M, et al. The heart, macrocirculation and microcirculation in hypertension: a unifying hypothesis. J Hypertens. 2003;21: S19–S23.
- Struijker Boudier HA. From macrocirculation to microcirculation: benefits of preterax. Am J Hypertens. 2007;20:S15–S18.
- Lee ML, Rosner BA, Weiss ST. Relationship of blood pressure to cardiovascular death: the effects of pulse pressure in the elderly. Ann Epidemiol 1999;9:101–107.
- Laurent S, Boutouyrie P. The structural factor of hypertension: large and small artery alterations. Circ Res. 2015;116(6):1007–1021.
- Laurent S, Agabiti-Rosei E. The cross-talk between the Macro- and Microcirculation. In: Nilson PM, Olsen MH, Laurent S. Early Vascular Aging (EVA). Elsevier Inc.;2015. p. 105–115.
- Rossi MA. Pathologic fibrosis and connective tissue matrix in left ventricular hypertrophy due to chronic arterial hypertension in humans. J Hypertens 1998;16:1031–1041.
- Ciulla M, Paliotti R, Hess DB, et al. Echocardiographic patterns of myocardial fibrosis in hypertensive patients: endomyocardial biopsy versus ultrasonic tissue characterization. J Am Soc Echocardiogr 1997;10:657–664.
- Angeli F, Reboldi G, Verdecchia P. Microcirculation and left-ventricular hypertrophy. J Hypertens. 2012;30(3):477–481.
- Wan SH, Vogel MW, Chen HH. Pre-clinical diastolic dysfunction. J Am Coll Cardiol. 2014;63(5):407–416.
- Świerblewska E, Wolf J, Kunicka K, et al. Prevalence and distribution of left ventricular diastolic dysfunction in treated patients with long-lasting hypertension. Blood Press. 2018;27(6):376–384.
- Wang L, Wong TY, Sharrett AR, et al. Relationship between retinal arteriolar narrowing and myocardial perfusion: multi-ethnic study of atherosclerosis. Hypertension. 2008;51(1):119–126. Epub 2007 Nov 12.
- Wong TY, Klein R, Sharrett AR, et al. Retinal arteriolar narrowing and risk of coronary heart disease in men and woman: the Atherosclerosis Risk in Communities Study. JAMA 2002;287:1153–1159.
- Izzard AS, Rizzoni D, Agabiti-Rosei E, et al. Small artery structure and hypertension: adaptive changes and target organ damage. J Hypertens. 2005;23(2):247–50.
- Heagerty AM, Heerkens EH, Izzard AS. Small artery structure and function in hypertension. J Cell Mol Med. 2010;14(5):1037–1043.
- Rizzoni D, Palombo C, Porteri E, et al. Relationships between coronary vasodilator capacity and small artery remodeling in hypertensive patients. J Hypertens 2003;21:625–632.
- Wong TY, Klein R, Sharrett AR, et al. Cerebral white matter lesions, retinopathy, and incident clinical stroke. JAMA 2002;288:67–74.
- Cheung N, Bluemke DA, Klein R, et al. Retinal arteriolar narrowing and left ventricular remodeling: the multi-ethnic study of atherosclerosis. J Am Coll Cardiol. 2007;50(1):48–55
- Tikellis G, Arnett DK, Skelton TN, et al. Retinal arteriolar narrowing and left ventricular hypertrophy in African Americans. the Atherosclerosis Risk in Communities (ARIC) study. Am J Hypertens. 2008;21(3):352–359.
- Wong TY, Rosamond W, Chang PP, et al. Retinopathy and risk of congestive heart failure. JAMA. 2005;293(1):63–69.
- Park JB, Schiffrin EL. Small artery remodelling is the most prevalent (earliest?) form of target organ damage in mild essential hypertension. J Hypertens. 2001;19:921–930.
- Agabiti-Rosei E, Rizzoni D. Microvascular structure as a prognostically relevant endpoint. J Hypertens 2017;35:914–921.
