Abstract
In this report, we present a challenging case of a 63-year-old Caucasian woman with an extreme stress response to blood pressure (BP) measurements. Office- and ambulatory BP measurements consistently found systolic BP above 200 mmHg. However, antihypertensive medication made her dizzy and extremely unwell, and she could barely tolerate treatment with a moderate dose of angiotensin-converting enzyme (ACE) inhibitor. Finger-cuff-based measurements (Finapres Finometer®) revealed extreme hypertension in relation to contact with medical professionals, but hypotension when the patient was seated alone unobserved. Months after, the patient suffered a hemorrhagic stroke possibly related to her extreme BP-fluctuations in stressful situations.
Introduction
White-coat hypertension (WCH) is the condition where blood pressure (BP) is elevated when measured in the doctor’s office, but within the normal range during ambulatory- or home BP measurements (ABPM and HBPM, respectively) [Citation1]. The prevalence of WCH has been estimated to be as much as 40% of the office hypertensive population [Citation1] with the highest prevalence in women, non-smokers, older patients, and patients with an office systolic BP between 140–159 mmHg [Citation2,Citation3]. Widely believed to be psychogenic in nature, WCH is associated with patient anxiety levels and increased sympathetic activity [Citation4,Citation5]. It has been proposed that the diagnosis of WCH should be based on office readings above 140/90 mmHg and a normal out-of-office BP [Citation1]. Both the prognosis and the treatment of the condition is controversial and most studies on WCH have been conducted in patients with mild to moderate WCH.
In this report, we present a challenging case with most unfortunate health consequences for a 65-year-old woman with an extreme BP rise during cuff BP-measurements and in the presence of health professionals.
Case description
A 63-year-old Caucasian woman was referred to our cardiology department from a private sector cardiology clinic, with presumed WCH. Despite treatment with an angiotensin-converting enzyme (ACE) inhibitor (ramipril) at half of maximum recommended dose, the patient had persistent hypertension with systolic BP >200 mmHg in the office as well as during HBPM and 24 h-ABPM. She could not tolerate any additional medical antihypertensive therapy. ACE-inhibitor in maximum dose, as well as the addition of calcium channel blockers and diuretics had all resulted in severe dizziness and discomfort. Echocardiography, urine samples and biochemistry did not indicate hypertension-mediated organ damage (HMOD) and the patient did not have a family history of premature cardiovascular disease (CVD). At the time of referral, neither the patient’s general practitioner (GP), nor the referring cardiology-specialist had any further diagnostic or treatment options. When in contact with medical professionals, the patient was consistently found to be extraordinarily anxious, which could to some degree have been caused by a previous traumatic experience in hospital. The patient rejected any suggestion of further upper arm-cuff-based measurements of any kind, claiming that these types of measurements had caused unacceptable discomfort to her previously. The patient did, however, agree to have measurements conducted using a Finapres Finometer® device. This device uses a small finger-cuff to conduct continuous beat-by-beat BP-measurements and gives waveform measurements similar to intraarterial recordings [Citation6]. To minimize the impact of potential WCH, the measurements were conducted with the patient seated alone and unobserved, with the nurse checking in on the patient on occasion. The measurements from the patient can be seen in .
Figure 1. Diagram showing the continuous BP-measurements of the cuff-less Finapress Finometer® BP-device. A: Spontaneous initiation of breathing exercises by the patient. B: The patient starts to report increasing discomfort from the finger-cuff. C: The nurse conducts a single BP-measurement using the upper arm cuff. D: The nurse re-enters the room and informs the patient that the measurements will be finished soon.
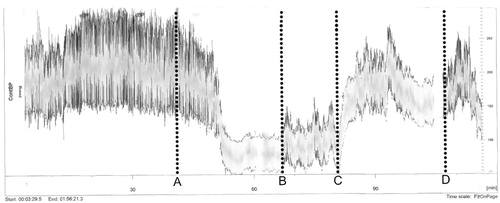
Initial measurements found a sustained increased BP of 250–300/150–160 mmHg.
After 50 min the patient decided to make a deliberate effort to calm down and she began taking deep low-frequent breaths. This resulted in a substantial decrease in BP to 100/50 mmHg, which was sustained for 15 min. At this time, she reported that she felt relaxed – “as at home”. After an additional 30 min, the patient called the nurse to report increasing discomfort from the finger-cuff. BP now increased to 125/60 mmHg. Seeing the significant decrease in the BP measured on the Finapres device compared to the initial measurements, the nurse conducted a single BP measurement using an oscillometric BP-device with a brachial cuff. During this measurement, the BP measured with the Finapres device increased dramatically and stabilized around 230/120 mmHg. The nurse once again left the room. BP slowly decreased during the following 30 min and then, again, increased abruptly as the nurse re-entered the room. At this point, the nurse informed the patient that the measurements would be over in 5 min, after which the BP once again stabilized at 150/100 mmHg.
On the basis of these findings, the lack of HMOD, as well as the highly elevated ABPM and HBPM gathered previously from the GP and cardiology specialist, the patient was diagnosed with a severe case of white coat effect and WCH was considered likely. Due to the problems observed in relation to previous cuff-based ABPM and the patient’s refusal to conduct further ABPM or HBPM, confirming the suspected WCH diagnosis according to ESH guidelines was not possible.
Antihypertensive treatment with the same ACE-inhibitor in the same dose was continued, as this was the only treatment the patient tolerated. The patient agreed to follow-up visits with finger-BP-measurements every two years. To avoid confusion, the patient agreed, that no BP measurements should be conducted by the patient or the patient’s GP in the time between ambulatory visits to our clinic.
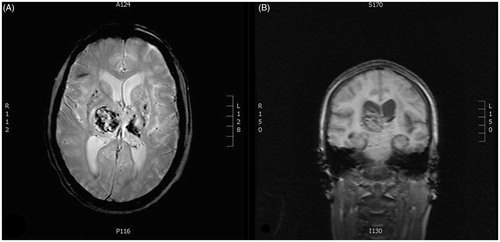
A few months later, the patient was admitted to the Neurological Department with suddenly developed headache, left sided hemiparesis and aphasia. An acute MRI was performed showing acute intra-cerebral hemorrhage in the right thalamic region (). The patient suffered severe neurological sequelae with persistent hemiparesis and speech-impediment.
Figure 2. Magnetic resonance images of the cerebrum of the patient after admission to the neurological department. The images reveal acute intracerebral hemorrhage in the right thalamic region as well as several microbleeds. A: Transverse plane. B: Coronal plane.
Since her stroke, the patient has been followed in our clinic with office finger-BP-measurements using a SOMNOtouch® non-invasive BP-device, as cuff based measurements are still not useful in this patient. In the first two years following the stroke, the patient’s BP increased and she was judged to both need and tolerate 3-drug antihypertensive therapy. Recently, the patient’s BP has markedly decreased during finger-BP measurements and at present the patient’s antihypertensive treatment regimen is exactly the same as prior to her stroke, as uptitration causes the symptoms of hypotension to reappear.
Discussion
This case illustrates many of the challenges facing the clinician when dealing with a patient who clearly displays symptoms of hypotension during therapy, but is also found to have severe hypertension when BP is measured.
Identifying WCH in the severely hypertensive patient
Our patient displayed several clues that the abnormally high measured BP could, at least in part, be due to a significant white-coat effect.
The patient reported no headache, problems with vision or any other subjective discomfort that could be associated with a high BP. Paraclinical examination was without signs of HMOD and the patient did not suffer from any known hypertension-related diseases, further decreasing the likelihood of sustained severe hypertension [Citation1].
In this patient, the chosen modality for BP measurement impacted significantly upon the findings. For more than three decades, finger arterial BP devices (e.g. Finapres Finometer®) have been used in both clinical practice and in clinical studies and the devices have been thoroughly validated [Citation6]. While office BP, HBPM and ABPM had all measured a very high BP, the finger-cuff-measurements exposed the complexity of the patient’s cardiovascular status, likely revealing her true everyday life resting BP. In our tertiary hypertension clinic, we have observed repeatedly, that in some hypertensive patients who suffer from orthostatic hypotension and show no signs of HMOD, BP-measurement with devices without a brachial cuff can be a valuable tool to reveal the patients true resting BP and the reason for antihypertensive drug intolerance. Furthermore, this case clearly illustrates the impact of attended versus unattended BP measurements, a matter that has recently been extensively debated in relation to the publication of the SPRINT trial [Citation7].
Prognosis and treatment of WCH
It seems reasonable to ask if the tragic outcome for our patient could be related to her suspected WCH. However, several large studies have found no significant increase in cardiovascular mortality for WCH-patients compared to sustained normotensive patients [Citation8]. The largest and most recent meta-analysis evaluating CVD risk in patients with WCH did, however, find that untreated WCH-patients had an increased cardiovascular risk compared to sustained normotensive patients [Citation9]. Antihypertensive treatment of WCH patients is controversial as two large meta-analysis found treatment to respectively increase [Citation10] and decrease [Citation9] CVD risk in this patient group. Current ESH/ECS guidelines recommend medical treatment of WCH only in patients with increased CVD risk or HMOD, while American guidelines do not recommend pharmacological treatment of WCH [Citation1,Citation11]. Due to unacceptable symptoms of hypotension whenever antihypertensive treatment was intensified, no changes in our patient’s antihypertensive treatment were made.
BP-variability and anxiety as cardiovascular risk factors
Several studies have found variation in both visit-to-visit-, day-to-day- and 24 h BP to be associated with an increased incidence of cardiovascular morbidity and mortality [Citation12–14]. Naturally, a single office-BP does not provide any information on BP-variability, which may be severely underappreciated in the clinic [Citation13]. Based on all the BP-measurements conducted in our patient, she displayed a high BP-variability and she might therefore have been at a much higher cardiovascular risk than expected from the lowest measured BP. This may apply to many patients with a significant white coat effect. According to American- and ESH/ECS guidelines BP variability is, however, not recommended treated, when the average resting BP is acceptable [Citation1,Citation11].
The patient’s anxiety could also have played a role in both the clinical findings and the clinical outcome. A meta-analysis of 12 prospective studies with a minimum follow-up of 6 months found anxiety to be associated with all-cause mortality, as well as cardiac mortality and new cardiac events [Citation15]. However, a recent large cohort study did not find a long term increased risk of stroke in patients suffering from anxiety during 32.720 years of follow-up [Citation16]. It may be, that anxiety is a separate risk factor for WCH patients leading to a worse clinical outcome. However, to our knowledge, so far, no studies on this particular question have been published.
Conclusion
Measuring the true resting BP can often be a clinical challenge. In patients who at the same time present with high BP and symptoms of hypotension on antihypertensive treatment and where there are no signs of HMOD, measurements with devices without an upper-arm cuff may be a helpful tool to gain insight into the true nature of the patient’s resting BP.
Studies of WCH mostly involve patients with mild to moderate WCH. As the presented case illustrates, severe WCH with high BP variability may be less benign, and in addition to medical therapy, early referral to psychologist or psychiatrist could be considered, if there is reason to suspect that the patient is under severe stress or suffers from extreme anxiety.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;1:3021–3104.
- Dolan E, Stanton A, Atkins N, et al. Determinants of white-coat hypertension. Blood Press Monit. 2004;9:307–309.
- Manios ED, Koroboki EA, Tsivgoulis GK, et al. Factors influencing white-coat effect. Am J Hypertens. 2008;21:153–158.
- Grassi G, Turri C, Vailati S, et al. Muscle and skin sympathetic nerve traffic during the “white-coat” effect. Circulation. 1999;100:222–225.
- Jhalani J, Goyal T, Clemow L, et al. Anxiety and outcome expectations predict the white-coat effect. Blood Press Monit. 2005;10:317–319.
- Schutte AE, Huisman HW, van Rooyen JM, et al. Validation of the Finometer device for measurement of blood pressure in black women. J Hum Hypertens. 2004;18:79–84.
- Kjeldsen SE, Lund-Johansen P, Nilsson PM, et al. Unattended blood pressure measurements in the systolic blood pressure intervention trial. Hypertension. 2016;67:808–812.
- Fagard RH, Cornelissen VA. Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: a meta-analysis. J Hypertens. 2007;25:2193–2198.
- Huang Y, Huang W, Mai W, et al. White-coat hypertension is a risk factor for cardiovascular diseases and total mortality. J Hypertens. 2017;35:677–688.
- Franklin SS, Thijs L, Hansen TW, et al. Significance of white-coat hypertension in older persons with isolated systolic hypertension: a meta-analysis using the International Database on Ambulatory Blood Pressure Monitoring in Relation to Cardiovascular Outcomes population. Hypertension. 2012;59:564–571.
- Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71:e127–e248.
- Kikuya M, Ohkubo T, Metoki H, et al. Day-by-day variability of blood pressure and heart rate at home as a novel predictor of prognosis: the Ohasama study. Hypertension. 2008;52:1045–1050.
- Stevens SL, Wood S, Koshiaris C, et al. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ. 2016;356:i4098
- Rothwell PM, Howard SC, Dolan E, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;13:895–905.
- Roest AM, Martens E, de Jonge P, et al. Anxiety and risk of incident coronary heart disease: a meta-analysis. J Am Coll Cardiol. 2010;29:38–46.
- Portegies ML, Bos MJ, Koudstaal PJ, et al. Anxiety and the risk of stroke: the Rotterdam study. Stroke. 2016;47:1120–1123.
