Abstract
Purpose: Mouthwash is used by a large population. Short-term clinical trials have shown that antibacterial mouthwash deplete oral nitrate-reducing bacteria, and decrease systemic nitric oxide bioavailability. Our previous publication from the San Juan Overweight Adults Longitudinal Study (SOALS) was the first to show frequent over-the-counter mouthwash use was independently associated with increased risk of prediabetes/diabetes. This manuscript evaluates whether over-the-counter mouthwash was associated with increased risk of hypertension.
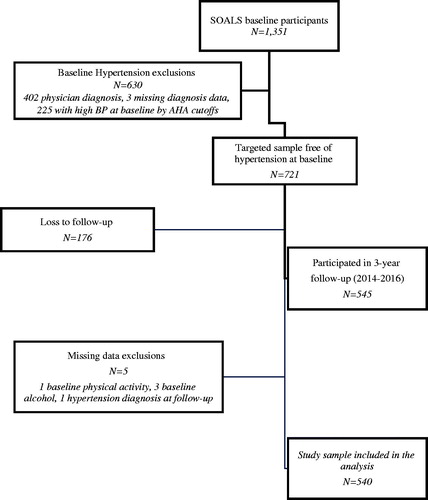
Materials and methods: SOALS recruited 40-65 year old overweight/obese individuals; baseline evaluations started in 2011 and the 3-year follow-up exam was completed by 2016. From the 1028 participants (76%) who completed follow-up, we excluded people with reported physician diagnosis of hypertension or systolic or diastolic BP at or above the hypertension cut-offs (n = 481), missing smoking (n = 1), missing physical activity (n = 1) and missing alcohol intake (n = 5) at baseline; 540 participants were included. The primary exposure was mouthwash use twice daily or more. The primary outcome for this manuscript is self-reported physician-diagnosed hypertension over the follow-up. We used Poisson regression controlling for age, sex, smoking, physical activity, waist circumference, alcohol intake, systolic blood pressure, pre-diabetes/diabetes status and cardiac medication use. We additionally evaluated other mouthwash use categorizations.
Results: Twelve percent (66/540) developed hypertension over follow-up. People who used mouthwash twice/day or more had higher incidence of hypertension compared to less frequent users (Incidence Rate Ratio = 1.85; 95% Confidence Interval: 1.17, 2.94), and compared to non-users (IRR = 2.17; 95% CI: 1.27, 3.71). Several additional potential confounders evaluated did not impact these associations. Associations persisted among never smokers. Additional outcomes including BP assessed at a single study visit did not show associations.
Conclusion: In this study, frequent regular use of over-the-counter mouthwash was associated with increased risk of hypertension, independent of major risk factors for hypertension and several other potential confounders.
Introduction
Hypertension is a leading preventable risk factor for premature death and disability worldwide [Citation1]. Identifying new modifiable factors would help reduce the global burden of hypertension. Lower nitric oxide (NO) bioavailability is associated with hypertension [Citation2,Citation3] via direct loss of a vasodilator and secondary effects that increase oxidative stress and inflammation, which could lead to vasculature injury and hypertension [Citation4].
Recent studies suggest oral microbes as important modulators of systemic NO-bioavailability. Nitrate is reduced by oral nitrate reductase containing bacteria to nitrite, which upon swallowing stimulates NO-signaling. Antibacterial mouthwash is known to reduce oral bacteria and gingivitis [Citation5–7], but the detrimental systemic impact of mouthwash on endogenous and exogenous NO synthesis and blood pressure (BP) has only been evaluated in small, short-term clinical trials [Citation8–14]. Mouthrinsing with Chlorhexidine ≥ twice daily (a common prescription bactericidal mouthwash), abolished the effects of exogenous nitrate-dependent increases in plasma and salivary nitrate and nitrite, increasing BP among hypertensive and normotensive individuals [Citation8–14]. A weaker antibacterial over-the-counter mouthwash also reduced plasma nitrite levels [Citation12,Citation13]. These studies suggest that disruption of oral microflora by antibacterial mouthwash may have detrimental health effects by lowering NO bioavailability.
Most prescription as well as over-the-counter mouthwashes, including fluoride mouthwash for caries reduction, have bactericidal ingredients. In 2017, 203 million (62%) Americans used mouthwash/dental rinse [Citation15], and 17 million used it ≥ twice daily [Citation16]. Almost two-thirds of a representative sample in the US used mouthwash to treat dental disease or dental problems in the last seven days, and 36% used it daily [Citation17].
Most studies to date have evaluated short-term effects of mouthwash on BP. Potential adverse effects of chronic use have been discussed [Citation18]. Our recent publication [Citation19] was the first to suggest a detrimental systemic impact of chronic mouthwash use; over-the-counter mouthwash use ≥ twice daily was associated with 55% increased risk for development of pre-diabetes/diabetes over a 3-year follow-up period. Hence, we evaluated whether routine over-the-counter mouthwash use increases hypertension risk.
Methods
Participants
University of Puerto Rico Human Research Subjects Protection Office Institutional Review Board approved the study and all participants gave informed consent. This study is reported following STROBE Guidelines. SOALS is a longitudinal study conducted among non-institutionalized Hispanic adults, recruited primarily from San Juan metropolitan area using flyers and other means. Recruitment and baseline data collection started in 2011 and the 3-year follow-up exam was completed in 2016. Eligibility criteria for this cohort study include: (1) age 40-65 years, (2) overweight/obese (body mass index ≥ 25.0 kg/m2), (3) free of clinically diagnosed diabetes prior to the baseline exam. Baseline exclusion criteria are: (1) physician-diagnosed diabetes or taking either insulin or oral anti-hyperglycemic agents; (2) pregnancy; (3) hypoglycaemia; (4) cardiovascular and other severe health conditions or psychological or physical disabilities that would interfere with participation in the study; and (5) plans to relocate in the subsequent three-year period [Citation20]. Retention efforts included phone calls, letters and tokens. The follow-up exam had similar data collection procedures. We used similar methods as our prior publication [Citation19] relating mouthwash and pre-diabetes/diabetes.
Mouthwash use assessment
Baseline and follow-up questionnaires assessed frequency of oral hygiene aids including mouthwash use (not limiting brands or types). For mouthwash users, we subsequently gathered details about their mouthwash use through a phone interview; 70% responded. The primary exposure was defined as mouthwash use ≥ twice daily, to be consistent with other publications and with the generally advertised/recommended frequency [Citation9,Citation10,Citation19,Citation21]. We additionally evaluated other categorizations of mouthwash use.
Blood pressure assessments
Nurses were trained utilising audiovisual techniques to minimise observer bias, and double stethoscope to reduce within and between observer variability [Citation22]. Calibration was conducted by an experienced trainer using the double stethoscope simultaneously; concordance within 4 mmHg was achieved. Re-training was conducted as necessary. The gold standard Korotkoff auscultatory method with a mercury sphygmomanometer was utilised to assess BP after 5 min of rest in a quiet and relaxing setting [Citation23]. Three repeat measurements (1 min between measures) were conducted in the upper right arm in a sitting position and averaged to compute mean SBP and DBP.
Hypertension classification
Participants reported physician diagnosis and prescribed medications for hypertension or high BP. We defined the primary outcome as reported physician-diagnosed hypertension over the three-year follow-up. Since the classifications of BP changed in 2017, and the study assessments were conducted between 2011 and 2016, we presumed that physicians who may have evaluated our participants used the prior classification during the time covering SOALS assessments. Hence, we chose to use the prior hypertension status cut-offs [Citation24] for SBP and DBP.
As secondary outcomes, we evaluated change in continuous SBP and DBP (follow-up minus baseline). For these analyses, we additionally excluded people who reported physician-diagnosed hypertension during the follow-up, as treatment or lifestyle changes may reduce SBP and DBP at follow-up. We defined an additional combined secondary outcome as development of pre-hypertension/hypertension. We considered participants free of hypertension at baseline to have progressed to hypertension if they reported physician-diagnosed hypertension over the follow-up or had average SBP ≥ 140 or DBP ≥ 90 (mmHg) at follow-up. We considered participants without diagnosed hypertension and with normal range of BP values at baseline (SBP < 120 and DBP < 80 mmHg) to progress to pre-hypertension if their average follow-up measures increased to pre-hypertensive levels (120 ≤ SBP ≤ 139 or 80 ≤ DBP ≤ 89). Since BP medications could be prescribed for reasons other than hypertension or high BP, we did not consider people who used medications in the absence of hypertension diagnosis as having hypertension.
Covariate assessment
Trained interviewers administered questionnaires to collect information on important potential confounders such as age, sex, smoking, physical activity, alcohol intake, and sleep breathing disorders. Dietary factors were assessed from food frequency questionnaires adapted for Puerto Ricans [Citation25], and nutrients were computed from these data. Anthropometric measurements were taken according to NHANES III procedures [Citation26]. Waist circumference was measured with a Gulick tape. Fasting high-sensitivity C-reactive protein (Hs-CRP) values were measured by high sensitive latex turbidimetric method AU5421 K-assay (Beckman Coulter, Inc., Brea, CA, USA). Trained and calibrated dentists performed the oral examination, and periodontal disease was defined using the CDC/AAP definition [Citation27,Citation28].
Analyses methods
Descriptive analyses are presented by mouthwash use. We compared the people who had complete follow-up data against the target sample (Appendix Table 1). We used Poisson regression models to evaluate the association between baseline mouthwash use and development of hypertension during the three-year follow-up. Time between baseline and follow-up visits was included in the models as an offset. When Poisson regression is applied to binomial data, the error for the estimate is overestimated [Citation29], which may be rectified by using sandwich estimation as robust error variance procedure [Citation30]. Hence we analysed the binary outcome using Poisson models with robust standard errors, and obtained Incidence Rate Ratios (IRRs) and 95% confidence intervals [Citation31]. We used linear regression for continuous SBP and DBP change outcomes. We controlled for age, sex, smoking, physical activity, waist circumference, alcohol intake (grams/day), SBP, pre-diabetes/diabetes status, and for cardiac medications used to treat conditions other than hypertension. Potential confounders that resulted in >10% change in the effect estimate for the primary analyses (mouthwash use and development of pre-hypertension/hypertension) were included in the final model. Additional secondary outcomes were evaluated using similar procedures.
For the primary analyses, we also explored similar models to assess the associations among sub-groups defined by baseline age, sex, smoking, physical activity, BMI, SBP, and DBP. Analyses were conducted using Stata 13.1.
Results
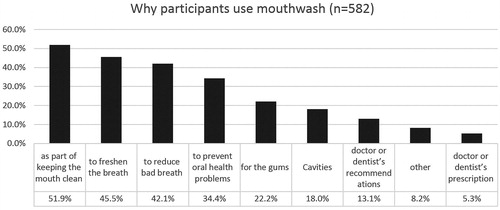
A total of 1351 completed the baseline study visit, 630 were excluded for high blood pressure at baseline. From the remaining 721, 176 (25%) did not complete the 3-year follow-up exam, and 5 were excluded for missing data (); remaining 540 were included in the analysis. The mean follow-up in this cohort was 2.97 years (SD = 0.24, range: 2.26–4.48, median 2.96 years). Participants reported their race as Mixed (61%), White (25%) and Black (14%). Participants using mouthwash ≥ 2/day () had lower income, were less educated, had more insulin resistance and diabetes, were less likely to add salt and had higher BP medication use. Participants included in the analyses were very similar to the targeted sample except that they had less active caries than the targeted sample (Appendix Table 1). At baseline, 7% used mouthwash > twice/day, 16% twice/day, 20% once/day, 11% less frequently and 46% never used mouthwash. At the 3-year follow-up, 69% of those who used mouthwash ≥ twice/day at baseline still did, and 73% who used ≥ once/day at baseline, still did. The subgroup of mouthwash users who responded to supplemental questionnaires, had used mouthwash on average for 16 years (SD = 14.8, range 0.08–60 years); 14% started using mouthwash before adulthood. Less than 20% reported using mouthwash because of health professionals’ recommendations; the majority used it as part of routine oral hygiene (85% after brushing and 10% before brushing). When asked to mark all their reasons for using mouthwash (), the most common reasons reported were to keep the mouth clean (49%), to freshen breath (43%) and to reduce bad breath (38%). Majority (64%) reported regularly using brands or types beyond their current mouthwash. Almost all (99%) brands or types that were confirmed (through reading label details or with an image sent as requested) included antibacterial ingredients.
Table 1. Baseline descriptive data by mouthwash use at baseline, among people free of diagnosed hypertension (mean ± SD or %).
In unadjusted models, using mouthwash ≥ twice daily was associated with an increased risk of physician diagnosed hypertension (IRR = 1.87, 95% CI: 1.07, 3.27), compared to no use. Using mouthwash once daily was associated with increase in SBP over follow-up (β = 3.98, 95% CI: 0.83, 7.13) compared to no use. Mouthwash use once daily (β = 2.31, 95% CI: 0.24, 4.38) and ≥ twice daily (β = 2.29, 95% CI: 0.30, 4.27) compared to no use are associated with increased DBP.
The final multivariate models relating mouthwash use and high SBP () controlled for age, sex, smoking status, physical activity (METs/week), waist circumference, pre-diabetes/diabetes, alcohol, BP medications and SBP (DBP models were adjusted for DBP instead). Adjusting for BMI instead of waist did not change the estimates. Additional potential confounders evaluated included: income, education, sleep breathing disorders, oral conditions (periodontal pocket depth, attachment loss, dental plaque scores, bleeding on probing, and dental caries), alcohol, dietary factors (high nitrate vegetables: beetroot and green leafy vegetables, total vegetables, total fruit and vegetable intake, added salt, coffee), nutrients (sodium, potassium, dietary fibre, polyunsaturated fatty acid) [Citation32], weight loss during follow-up, and DBP instead of SBP. None of these changed the effects estimates by more than 10%; hence were not included in the final models. shows the key results. People who used mouthwash ≥ twice/daily showed higher incidence of physician-diagnosed hypertension (IRR = 1.85, 95% CI: 1.17-2.94) compared to less frequent users, and compared to non-users (IRR = 2.17, 95% CI: 1.27-3.71). Compared to non-users, people who used mouthwash once daily showed significantly higher increase in SBP over the follow-up (β = 3.60, 95% CI: 0.52–6.68). Additional analyses (not shown in tables) comparing ≥ 1/day versus never use showed significant associations for the physician-diagnosed hypertension (IRR = 1.72, 95% CI: 1.06-2.77) as well as for DBP change (β = 1.72, 95% CI: 0.20–3.25). Other associations were in a similar direction but most were not significant.
Figure 3. Multivariate Poisson regression associations between mouthwash use and physician-diagnosed hypertension over the follow-up. (a) Multivariate associations between people who used mouthwash at different frequencies and physician-diagnosed hypertension compared to people who never use mouthwash; (b) multivariate associations between people who used mouthwash ≥ twice/daily and physician-diagnosed hypertension compared to less frequent users.
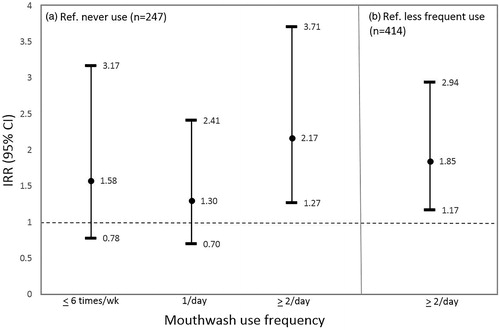
Table 2. Associations between mouthwash use and hypertension related outcomes over the follow-up§.
Subgroup analyses relating mouthwash use ≥ twice daily with physician-diagnosed hypertension are shown in . The IRR were twice as high among males (IRR = 3.96, 95% CI: 1.00–15.64) compared to women (IRR = 1.63, 95% CI: 0.99–2.71). Also, significant associations were present among < 55 year-olds, males, never smokers, people with obesity, pre-hypertensive levels of SBP and DBP, metabolic syndrome, high HOMA-IR, moderate/severe periodontal disease, people meeting physical activity recommendations, not currently drinking alcohol, and never adding salt to the food at the table (IRRs: 1.79–3.96). Although the number was small to compare subgroups by cardiac medications, the associations were persistent among people without cardiac medications (1.75).
Table 3. Hazard Ratios relating mouthwash use (≥twice daily vs less frequent use) and incidence of hypertension during the follow-up within subgroups defined by baseline characteristics§.
Discussion
People who used over-the-counter mouthwash ≥ twice/daily at baseline had 85% higher risk of physician-diagnosed hypertension compared to less frequent users, and more than twice that of non-users, over the 3-year follow-up of people free of hypertension at baseline. These associations were independent of major hypertension risk factors, and several additional potential confounders including dietary factors. The results remained significant and stronger among never smokers, suggesting that the associations were independent of confounding by smoking. The associations observed are for any mouthwash use including a range of different formulations and purposes. Most participants reported using mouthwash to keep the mouth clean and to freshen or improve the breath, and most reported using more than one brand. Since 99% of mouthwash contain some antibacterial ingredient, almost all types/brands are likely to have had a detrimental impact on nitrate reducing bacteria, which may increase BP.
Study measures assessed at a single visit would misclassify hypertension. Ambulatory BP monitoring (ABPM), which plays a critical role as a confirmatory tool for diagnosing masked as well as uncontrolled hypertension [Citation33–35] was not common practice at the time SOALS data was collected, and is not practical in large scale epidemiologic studies, hence was not performed. People with diagnosed hypertension may be on treatment to lower their BP making their study-assessed status erroneously classified as normotensive/pre-hypertensive. Given these limitations of BP measures in a single study visit, we used physician-diagnosed hypertension as the primary outcome instead of outcomes based on study measurements. The 2017 thresholds are lower than the prior thresholds, and some cases that meet 2017 thresholds would be missed in the physician diagnosis. Such misclassification is expected to be random with respect to mouthwash use, and hence likely to attenuate effect estimates. We additionally evaluated continuous measures of changes in study assessed SBP and DBP between baseline and follow-up visits, and combined physician-diagnosed and study assessed measures for the development of pre-hypertension/hypertension. None of these outcomes showed consistent associations with mouthwash use. Although the associations for these outcomes are in the expected direction, they are generally weaker compared to physician-diagnosed hypertension, likely due to the limitations of study assessed BP at a single visit. Our findings relating mouthwash use to pre-diabetes/diabetes (assessed from fasting and post-load glucose measures that are less affected by variability and settings), were robust using study measured or physician diagnosis of diabetes.
Given that hypertension and diabetes share several risk factors, it is not surprising that our results relating over-the-counter mouthwash use with increased risk of hypertension and pre-diabetes/diabetes [Citation19] in the same population are similar. No other study to date has evaluated the systemic impact of over-the-counter mouthwash. Although periodontal disease has been associated with both diabetes and hypertension [Citation36], mouthwash use is not associated with periodontitis in our study (see ), nor is there evidence for mouthwash to reduce periodontitis. Hence, the association of mouthwash use with increased diabetes and hypertension is likely to be mediated by different pathways. Routine mouthwash use may likely play a role in the development of both these conditions through the common pathway of inhibiting NO bioavailability. Antibacterial mouthwash is known to reduce oral bacteria and gingivitis [Citation5–7]. The detrimental systemic impact of mouthwash has been demonstrated in small, short-term clinical trials which show inhibited conversion of nitrate to nitrite and increased BP [Citation8–14,Citation37]. Nitrate is reduced by oral nitrate reductase containing bacteria to nitrite, which upon swallowing stimulates NO-signaling. Mouthrinsing with Chlorhexidine ≥ twice daily abolished the effects of exogenous nitrate-dependent increases in plasma and salivary nitrate and nitrite, and increased BP among hypertensive and normotensive individuals [Citation8–14]. A weaker antibacterial over-the-counter mouthwash also reduced plasma nitrite levels [Citation12,Citation13].
Mouthwash use was associated with increased hypertension across almost all subgroups, showing robustness and consistency of the findings. The potential impact of mouthwash on hypertension seems higher among subgroups without some hypertension risk factors, such as younger people, never smokers, people engaging in recommended levels of physical activity, not using alcohol, and never adding salt at the table. However, we also found stronger associations among individuals with obesity, higher SBP or DBP, metabolic syndrome, high HOMA-IR, and moderate/severe periodontal disease. Given the limited power of subgroup analysis, further studies are needed to verify whether the associations are due to chance, or truly vary across these factors.
A major limitation is that we cannot establish causality in this observational study. However, these results are supported by prior randomised controlled clinical trials. An additional limitation is that we did not directly assess oral nitrate reducing activity. Hence, we cannot directly attribute associations between mouthwash use and change in BP to mechanisms that mediate nitrate to nitrite reduction based on data from this study. However, we will be directly evaluating this in the future within the same population, and this pathway is supported by other literature including clinical trials.
Dietary nitrate is the substrate for oral nitrate reductase activity and nitrite formation, and vegetables are major source of nitrates. In addition to variations in intake of nitrate rich foods and beverages, nitrate content of produce varies according to the location and soil, making it difficult to estimate nitrate intake of individuals. Puerto Ricans are relatively homogeneous in generally having low intake of vegetables [Citation38], and controlling for various dietary factors (such as total and nitrate rich vegetables, potassium) did not change the effect estimates, thus reducing concern for confounding by dietary factors. The impact of mouthwash use on hypertension may likely be higher in populations with higher nitrate intake.
Mouthwash has evidence-based benefits and is therefore prescribed for individuals with specific conditions; however, prescriptions are for short-term use. On the contrary, over-the-counter mouthwash is advertised as part of routine oral hygiene, and commonly used long-term. The American Dental Association (ADA) gives a seal of acceptance to some commonly used mouthwash based on evidence of specific oral beneficial effects (e.g., gingivitis, halitosis or dental caries). While toxicology is evaluated as part of the criteria for the seal of acceptance, studies to date evaluating benefits and safety are mostly short-term. This is the first longitudinal study suggesting that routine long-term over-the-counter mouthwash use at the advertised/recommended frequency (twice a day), may increase the risk for development of pre-hypertension/hypertension. Triclosan, an antibacterial active ingredient in some mouthwash, was recently removed from over-the-counter antiseptic hand wash by the FDA on the basis of no added benefit beyond washing hands with standard soap and water [Citation39]; it was also shown to have harmful effects on the environment and health [Citation40]. It is therefore crucial to understand the long-term oral and systemic effects of mouthwash use before promoting or engaging in long-term routine use. Large long-term randomised, blinded and prospective clinical trials would be needed to evaluate and compare the systemic impact of different formulations/types/brands of over-the-counter mouthwash in different populations.
In conclusion, our study shows a significant higher risk of hypertension among people who used mouthwash twice or more daily compared to less frequent or non-users. This association is independent of major risk factors for hypertension and several other potential confounders, but further studies are needed to infer causality.
Acknowledgements
The authors acknowledge Dr. Oelisoa M. Andriankaja, Dr. Maribel Campos, Ms. Tania Ginebra, Ms. Carla León, Dr. Nicole M. Yordan López, Ms. Yashira Maldonado, Dr. Sasha Martínez, Dr. Evangelia Morou, Mr. Hector Marrero, Mr. Javier Cevallos, Ms. Xiomara O’Farrill, Ms. Samantha Ordaz, Dr. Cynthia Pérez, Ms. Yadiris Santaella, Dr. María Trak, Dr. Katherine Tucker, Ms. Grace Vélez, Mr. José Vergara, Ms. Lay Wah, Dr. Juan Carlos Zevallos, and PRCTRC nurse (Ms. Barbara Guzman) who contributed to the conduct/oversight/planning of data collection/manuscript preparation for their help with the study.
Disclosure statement
The authors have no conflicts of interest to disclose in the R01DE020111.
Additional information
Funding
References
- O'Brien E, Pickering T, Asmar R, et al. Working Group on Blood Pressure Monitoring of the European Society of Hypertension International Protocol for validation of blood pressure measuring devices in adults [Practice Guideline]. Blood Press Monit. 2002;7(1):3–17.
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87(1):315–424.
- Sansbury BE, Hill BG. Regulation of obesity and insulin resistance by nitric oxide. Free Rad Biol Med. 2014;73:383–399.
- Green DJ, Dawson EA, Groenewoud HM, et al. Is flow-mediated dilation nitric oxide mediated?: a meta-analysis. Hypertension. 2014;63(2):376–382.
- Haraszthy VI, Sreenivasan PK. Microbiological and clinical effects of an oral hygiene regimen. Contemp Clin Trials Commun. 2017;8:85–89.
- Vlachojannis C, Al-Ahmad A, Hellwig E, et al. Listerine(R) products: an update on the efficacy and safety. Phytother Res. 2016;30(3):367–373.
- Marya CM, Taneja P, Nagpal R, et al. Efficacy of chlorhexidine, xylitol, and chlorhexidine. + xylitol against dental plaque, gingivitis, and salivary Streptococcus mutans load: a randomised controlled trial. Oral Health Prevent Dentistry. 2017;15(6):529–536.
- Shapiro KB, Hotchkiss JH, Roe DA. Quantitative relationship between oral nitrate-reducing activity and the endogenous formation of N-nitrosoamino acids in humans. Food Chemical Toxicol. 1991;29(11):751–755.
- Kapil V, Haydar SM, Pearl V, et al. Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free Rad Biol Med. 2013;55:93–100.
- Bondonno CP, Liu AH, Croft KD, et al. Antibacterial mouthwash blunts oral nitrate reduction and increases blood pressure in treated hypertensive men and women. Am J Hypertens. 2015;28(5):572–575.
- Govoni M, Jansson EA, Weitzberg E, et al. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide. 2008;19(4):333–337.
- McDonagh SW, Winyard PG, Vanhatalo A, et al. The effects of chronic nitrate supplementation and the use of strong and weak antibacterial agents on plasma nitrite concentration and exercise blood pressure. Int J Sports Med. 2015;36(14):1177–1185.
- Woessner M, Smoliga JM, Tarzia B, et al. A stepwise reduction in plasma and salivary nitrite with increasing strengths of mouthwash following a dietary nitrate load. Nitric Oxide. 2016;54:1–7.
- Sundqvist ML, Lundberg JO, Weitzberg E. Effects of antiseptic mouthwash on resting metabolic rate: a randomized, double-blind, crossover study. Nitric Oxide. 2016;61:38–44.
- Statista. U.S. population: Usage of mouthwash/dental rinse from 2011 to 2020 2018 [May 18, 2018]. Available from: https://www.statista.com/statistics/286902/usage-mouthwash-dental-rinse-us-trend/
- Statista. U.S. population: Number of uses of mouthwash/dental rinse within 7 days from 2011 to 2017 2018 [May 18, 2018]. Available from: https://www.statista.com/statistics/286904/usage-frequency-of-mouthwash-dental-rinse-in-the-us-trend/
- CDC. Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Questionnaire (or Examination Protocol, or Laboratory Protocol). Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention 2014. [04/03/2019]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/Search/variablelist.aspx?Component=Questionnaire&CycleBeginYear=2011
- Hezel MP, Weitzberg E. The oral microbiome and nitric oxide homoeostasis. Oral Dis. 2015;21(1):7–16.
- Joshipura KJ, Munoz-Torres FJ, Morou-Bermudez E, et al. Over-the-counter mouthwash use and risk of pre-diabetes/diabetes. Nitric Oxide. 2017;71:14–20.
- Andriankaja OM, Jimenez JJ, Munoz-Torres FJ, et al. Lipid-lowering agents use and systemic and oral inflammation in overweight or obese adult Puerto Ricans: the San Juan Overweight Adults Longitudinal Study (SOALS). J Clin Periodontol. 2015;42(12):1090–1096.
- McDonagh ST, Wylie LJ, Winyard PG, et al. The effects of chronic nitrate supplementation and the use of strong and weak antibacterial agents on plasma nitrite concentration and exercise blood pressure. Int J Sports Med. 2015;36(14):1177–1185.
- Perloff D, Grim C, Flack J, et al. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88(5)1):2460–2470.
- Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111(5):697–716.
- Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252.
- Palacios C, Trak MA, Betancourt J, et al. Validation and reproducibility of a semi-quantitative FFQ as a measure of dietary intake in adults from Puerto Rico. Public Health Nutr. 2015;18(14):2550–2558.
- CDC. Centers for Disease and Control (CDC). The Third National Health and Nutrition Examination Survey (NHANES III, 1988-94) Reference Manuals and Reports. Anthropometry Procedures Manual. 1996.
- Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78(7s):1387–1399.
- Eke PI, Page RC, Wei L, et al. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. 2012;83(12):1449–1454.
- Zocchetti C, Consonni D, Bertazzi PA. Estimation of prevalence rate ratios from cross-sectional data. Int J Epidemiol. 1995;24(5):1064–1067.
- Royall RM. Model robust confidence intervals using maximum likelihood estimators. Int Stat Rev. 1986;54(2):221–226.
- Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706.
- Zhao D, Qi Y, Zheng Z, et al. Dietary factors associated with hypertension. Nat Rev Cardiol. 2011;8(8):456.
- Fujiwara T, Yano Y, Hoshide S, et al. Association of cardiovascular outcomes with masked hypertension defined by home blood pressure monitoring in a Japanese general practice population. JAMA Cardiol. 2018;3(7):583–590.
- Banegas JR, Ruilope LM, de la Sierra A, et al. Relationship between clinic and ambulatory blood-pressure measurements and mortality. N Engl J Med. 2018;378(16):1509–1520.
- de la Sierra A, Banegas JR, Vinyoles E, et al. Prevalence of masked hypertension in untreated and treated patients with office blood pressure below 130/80 mm Hg. Circulation. 2018;137(24):2651–2653.
- Muñoz Aguilera E, Suvan J, Buti J, et al. Periodontitis is associated with hypertension: a systematic review and meta-analysis. Cardiovasc Res. 2019. doi:10.1093/cvr/cvz201.
- Bryan NS, Tribble G, Angelov N. Oral microbiome and nitric oxide: the missing link in the management of blood pressure. Curr Hypertens Rep. 2017;19(4):33.
- Colon-Lopez V, Banerjee G, Gertz AM, et al. Behavioral correlates of fruit and vegetable intake in Puerto Rico: results from the Health Information National Trends Survey. Puerto Rico Health Sci J. 2013;32(4):194–199.
- FDA. U.S. Food & Drug Administration (FDA). FDA issues final rule on safety and effectiveness of antibacterial soaps. 2016 [updated September 2, 2016;May 5, 2018]. Available from: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm517478.htm
- Halden RU, Lindeman AE, Aiello AE, et al. The Florence statement on triclosan and triclocarban. Environ Health Perspect. 2017;125(6):064501.
Appendix
Table A1. Baseline comparison of analytical sample (n = 540) vs analytical sample plus loss to follow-up (n = 716) (mean ± SD or %).