Abstract
Purpose: Advanced glycation end products (AGEs) are a heterogeneous group of highly oxidant compounds which can potentiate microvascular and macrovascular complications through the formation of irreversible cross-links between molecules in the basal membrane and also by engaging the receptor for AGEs (RAGE). Soluble receptor for AGEs (sRAGE) is suggested to have a protective role neutralizing the toxic action of AGEs. We aimed to investigate differences in plasma levels of sRAGE alongside with classic cardiovascular risk factors between offspring of patients with early onset of coronary heart disease (CHD) and healthy controls.
Materials and methods: In a cross-sectional design, we examined 114 adult offspring of patients with premature CHD and 194 controls. Concentrations of soluble RAGE were quantified by ELISA methods. Aortic PWV was measured using Sphygmocor device. Multivariate logistic regressions were used to compare differences between the offspring and controls.
Results: In the offspring group there were more men (p = 0.023), both groups had similar age (28.5 vs. 28.9 years; p = 0.51). After adjustment for covariates, we observed significantly higher aPWV (6.17 vs. 5.82 m s−1; p = 0.001) and lower sRAGE (1308.11 vs. 1475.59; p = 0.009) in the offspring group compared to controls. The significant determinants of the intergroup difference were sRAGE (p = 0.0017), aPWV (p = 0.011) and current smoking (p = 0.0053).
Conclusion: Offspring of patients with early onset of CHD compared to age-matched healthy controls had significantly lower sRAGE levels suggesting a shift in the oxidative balance between stressors and defence mechanisms that may influence a higher cardiovascular risk in the future. The measurement of sRAGE might be a valuable predictor for more precise stratification of cardiovascular risk.
Introduction
Cardiovascular disease is a major cause of mortality worldwide. Classic cardiovascular risk factors such as family history of premature coronary heart disease (CHD), hypertension, diabetes mellitus, dyslipidemia and cigarette smoking have been demonstrated to be significantly associated with early CHD [Citation1–3].
Advanced glycation end products (AGEs) are a heterogeneous group of highly oxidant compounds with pathological significance in diabetes and several other chronic diseases such as atherosclerosis, chronic renal failure and neurodegenerative diseases [Citation4–8]. AGEs potentiate microvascular and macrovascular complications through the formation of irreversible cross-links between molecules in the basal membrane and also by engaging the receptor for AGEs (RAGE). RAGE is a multiligand cell-surface protein, exists in numerous variants and forms. It is expressed on multiple cell types – smooth muscle cells, macrophages, endothelial cells, cardiomyocytes, epithelial cells and so forth [Citation9].
In our study we focussed on soluble RAGE (sRAGE) – the form of RAGE that is cleaved from cell-surface RAGE by matrix metalloproteinases [Citation10]. The sRAGE has a protective role. It probably acts as a decoy for RAGE ligands, preventing AGEs from reaching cellular receptors and neutralizing the toxic action of AGEs [Citation11].
Indeed, some authors have shown that sRAGE is a naturally occurring inhibitor of toxic impact caused by AGE-RAGE action. This favourable effect of sRAGE, particularly inhibition of atherosclerotic development and progression, was demonstrated in several animal models [Citation12,Citation13]. Already in the 90s, Park et al. demonstrated that mice treated daily with genetically engineered murine sRAGE suppressed diabetes associated acceleration of atherosclerosis, this effect was independent of glycemic and lipid status [Citation11].
There are few observational studies in humans. Decreased plasma levels of sRAGE were found in patients with essential hypertension [Citation14]. Moreover, decreased plasma levels of sRAGE were independently associated with increased stiffness of the elastic central arteries in a general population [Citation15]. On the other hand, high plasmatic concentrations of sRAGE were associated with a lower incidence of coronary artery disease in nondiabetic men [Citation16]. To our knowledge, no previous paper has investigated sRAGE concentration in adult offspring of patients with positive family history of premature myocardial infarction. Therefore, in present study, we aimed to investigate differences in plasma levels of sRAGE alongside with classical cardiovascular risk factors (including the presence of metabolic syndrome) between offspring of patients with early onset of coronary heart disease and healthy controls.
Patients and methods
Study subjects
We examined adult offspring of patients with premature coronary heart disease (i.e. myocardial infarction or angina pectoris with significant stenosis of the artery verified by coronary angiography, diagnosed before the age of 50 years). We selected CHD patients using a Clinical Information System of the University hospital in Pilsen. We found 657 patients with premature CHD who were hospitalized between 6 June 2003 and 6 May 2013. We addressed 648 of these patients in whom we assumed that they might have adult offspring. Offspring of 121 patients agreed to participate in the study. The inclusion criterion for offspring was age older than 18 years. No exclusion criteria were applied. In total, we included 114 offspring in present study.
Subjects in the control group had negative family history of cardiovascular disease. We included medical students (n = 92) and subjects from the population-based post-MONICA survey (n = 104) [Citation17]. Of 196 examined controls, two were excluded because of missing anthropometric data. Thus, total number of controls included in a final analysis was 194.
Examination
The research protocol included the administration of a standardized questionnaire to obtain information on each subject’s medical history, smoking habits, physical activity and use of medications.
Regular physical activity was defined as a moderate or vigorous activity (jogging, swimming) that is practiced two or more days per week for a minimum of 20 min.
Blood pressure was measured in triplicate in the right arm with the subject in the sitting position after at least 5 min at rest. Standard mercury sphygmomanometers and correctly sized cuffs were used. The participant’s right arm was supported at heart level. The maximum inflation level was determined before the actual measurement. Blood pressure values were recorded to the nearest 2 mmHg. The mean value of the last two readings was used for further analysis. Mean arterial pressure (MAP) derived from office blood pressure measurement was calculated as diastolic pressure plus one third of pulse pressure. Arterial hypertension was defined as elevation of systolic blood pressure ≥140 mmHg and/or diastolic pressure ≥90 mmHg and/or use of antihypertensive medication.
Height and body weight were determined for all participants. Body mass index (BMI) was calculated as body weight (kg)/height2 (m2). The waist circumference was measured at the midpoint between the lower margin of the last palpable rib and the top of the iliac crest, standing, at the end of gentle expiration. Because of the age of participants, we used stricter threshold for abdominal obesity which is equivalent to increased risk in Caucasians – waist circumference ≥94 cm in men, ≥80 cm in women.
Diabetes was defined as fasting plasma glucose ≥7.0 mmol L−1 or use of oral antidiabetic drugs and/or insulin. Impaired fasting glucose was defined as glucose levels of 5.6–6.9 mmol L−1.
Venous blood samples were drawn after at least 10 h overnight fasting and determined in the research laboratory of the Department of Immunodiagnostics and Biochemistry, University Hospital in Pilsen. Soluble RAGEs concentrations were quantified by ELISA methods using a Human RAGE Quantikine ELISA Kit (R&D Systems, Minneapolis, MN).
The metabolic syndrome was defined according to the modified NCEP criteria [Citation18], the presence of any three of the following five characteristics is required for a diagnosis: (1) arterial hypertension or antihypertensive drug treatment (blood pressure ≥130/≥85 mmHg); (2) pathological waist circumference (≥94 cm in men, ≥80 cm in women); (3) serum triglycerides ≥1.7 mmol L−1 or lipid-lowering drug treatment; (4) high density lipoprotein (HDL) cholesterol ˂1.0 mmol L−1 in men, ˂1.3 mmol L−1 in women; (5) fasting glycaemia ≥5.6 mmol L−1 or impaired fasting glucose or presence of type 2 diabetes mellitus.
All persons gave their informed consent prior to their inclusion into the study. The study was approved by the local ethics committee of the Faculty of medicine, Charles University in Pilsen, Czech Republic. The study was in accordance with the Declaration of Helsinki.
Arterial measurement
Large artery properties were measured using SphygmoCor device (AtCor Medical Ltd, West Ryde, New South Wales, Australia) in the recumbent position [Citation19]. Aortic pulse wave velocity (aPWV) was assessed according to recommendations [Citation20]. Consecutive registrations of the pulse waves are ECG-gated and thus, the time shift (Dt) between the foot of wave at the first and second site can be calculated. The distance between the two sites was measured on the body surface. To determine aPWV, we measured the distance from the jugular fossa to the pulsation of the femoral artery in the groyne and subtracted the distance from the jugular fossa to carotid pulsation in order to obtain the travelled distance (D). PWV was calculated as D (m)/Dt (s) [Citation21].
Statistical methods
For database management and statistical analyses, we used the SAS software, version 9.3 (SAS Institute, Cary, NC). Data were presented as mean ± SD or proportions.
A Student t test and χ2 test and Wilcoxon test were used to compare differences between the offspring and control group. For the purposes of regression analysis, non-normally distributed variables were normalized using logarithmic transformation. We used multivariate logistic regression to examine which variables determine differences between offspring and control group. For the purpose of regression analyses, we dichotomized following variables: LDL cholesterol >2.5 mmol L−1 vs. its lower values; aortic pulse wave velocity 4th quartile (e.g. ≥6.5 m s−1) versus others; ankle brachial index < 1 versus its higher values, and sRAGE 1st quartile (e.g. <1059 pg mL−1) versus others.
Results
The characteristics of the 114 offspring and 194 controls are summarized in . In offspring group there were more men than women compared to controls (49.1 vs. 35.6%, p = 0.023). Both groups had similar age (28.5 ± 6.4 vs. 28.9 ± 5.3 years; p = 0.51) and BMI (24.3 (21.6–26.9) versus 23.2 (20.9–26.6) kg m−2; p = 0.11).
Table 1. Characteristics of the study population.
Subjects in both groups had similar systolic and diastolic pressure (p = 0.13 and 0.06, respectively). However, offspring fulfilled criteria for arterial hypertension more frequently −16.7 versus 7.7%, p = 0.023. Antihypertensive drugs were used only in the offspring group (2.7 vs. 0%, p = 0.05).
None of the subjects had diabetes mellitus. Also values of fasting glycaemia were similar in both groups (p = 0.40). However, offspring had slightly higher prevalence of impaired fasting glucose (5.3 vs. 1.6%; p = 0.08; data not shown). All of the respondents had value of glycated haemoglobin (HbA1c) in the normal range, however in the offspring group we found higher level of HbA1c (34.6 ± 0.31 vs. 33.7 ± 0.29 mmol mol−1, p = 0.03). We did not observe significant differences either in serum insulin or in C-peptide levels. Also, prevalence of metabolic syndrome was similar in both groups (8.8 vs. 5.7%, p = 0.35).
Concerning the lipid metabolism, only LDL cholesterol was higher in offspring compared to controls (2.70 ± 0.9 vs. 2.46 ± 0.7 mmol L−1; p = 0.02). Offspring compared to controls more frequently reported history of smoking (50.9 vs. 33.0%, p = 0.003) or current smoking (37.7 vs. 24.7%; p = 0.020).
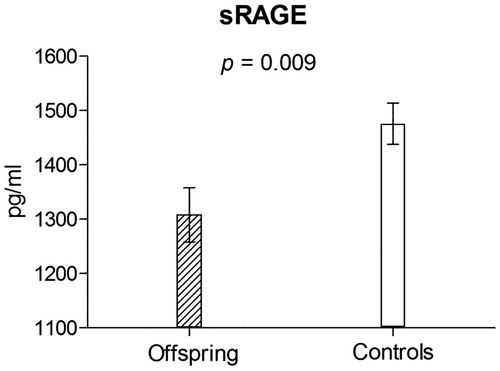
Offspring compared to control group had higher aortic PWV (6.2 ± 1.1 vs. 5.8 ± 1.0 m s−1; p = 0.002) and lower serum levels of sRAGE (1289.2 ± 518.3 vs. 1492.1 ± 537.8 pg mL−1; p = 0.001; ). In regression models adjusted for all relevant covariates these differences remained significant with p values ≤.009 ( and ).
Figure 1. Aortic PWV in the offspring and control group adjusted for major cofounders – results of the multivariate linear regression analysis.
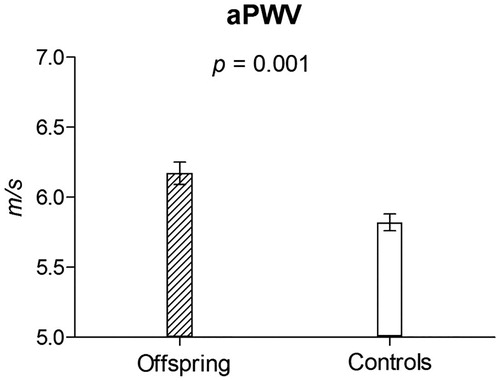
Figure 2. Soluble RAGE levels in the offspring and control group adjusted for major cofounders – results of the multivariate linear regression analysis.
shows correlation analysis among aortic PWV, sRAGE and other cardiovascular risk factors. We searched for determinants of the differences between offspring and controls using multiple logistic regression. As covariates we considered sex, age, systolic blood pressure, body mass index, serum glucose, serum LDL cholesterol, current smoking, aortic PWV, serum levels of RAGE and ankle brachial index (ABI). In the first step, we put all variables using their continuous values (if applicable). The significant determinants of the difference between offspring and controls were serum LDL cholesterol, current smoking, aPWV, sRAGE and ABI (data not shown). In the second step, we used dichotomized values for LDL, aPWV, sRAGE and ABI (). The results were confirmatory to the previous analysis, with the exception of ABI and LDL cholesterol, which were no longer significant determinants of the between-group difference.
Table 2. Correlation analysis between sRAGE, aortic PWV and other cardiovascular risk factors.
Table 3. Determinants of the difference between offspring and controls – results of multivariate logistic regression analysis.
In a next step we ran series of sensitivity regression models in which we one by one replaced serum glycaemia with serum insulin, BMI with waist to hip ratio or pathological waist and systolic pressure with diastolic pressure. Additionally, we added use of antihypertensive treatment into the model. In all these sensitivity analyses the main results remained virtually the same.
Furthermore, we analyzed men and women separately (Supplementary Tables 1 and 2). In women, both in analyses used continuous variables and their dichotomized counterparts, LDL cholesterol, smoking, PWV and sRAGE were independent determinants of differences between the two groups. However, in men we observed only borderline significance for smoking and PWV.
As smoking was reported to be an important factor affecting sRAGE values [Citation22] we ran another sensitivity analysis including only non-smokers. Supplementary Table 3 lists characteristics of offspring and controls in non-smokers. Results are comparable to the main results with the exception of body weight associated characteristics which were now more statistically significant. However, difference in aortic PWV and serum levels of sRAGE were significant. In multivariable adjusted regression, serum levels of sRAGE remained significant determinant of the difference between offspring and control group (Supplementary Table 4).
Discussion
To our knowledge this is the first study investigating soluble RAGE in offspring of patients with premature coronary heart disease. We observed that serum levels of sRAGE were significant independent determinants of the difference between offspring and control group even after adjustment for classical cardiovascular risk factors. Our finding is in agreement with the several previous studies reporting that AGE/RAGE system can be used for more precise cardiovascular risk stratification [Citation10,Citation12,Citation23–25]. The other significant determinants of the between-group difference were increased aortic stiffness, current smoking and to lesser extent LDL cholesterol.
Family history of CHD is considered to be a non-modifiable risk factor of future cardiovascular events. In the multi-ethnic study of atherosclerosis, Cohen et al. demonstrated that asymptomatic subjects with a zero coronary artery calcium score and a positive family history of CHD are at increased risk of future cardiovascular disease compared with subjects with negative history of CHD [Citation26]. In the present study we demonstrated significantly higher aortic stiffness (assessed as aortic pulse wave velocity) in the offspring group. PWV is a complex parameter that reflects damage of arterial wall caused by various factors, especially by blood pressure and age, but also by disturbances of glucose and lipid metabolism, renal function and so forth. Our finding is in accordance with the view that stiffening of central arteries may precede the onset of manifest atherosclerosis, thus being a potential target for early prevention of cardiovascular disease [Citation27]. As mentioned above, the AGEs/RAGE complex participates in various stages of atherosclerosis/arterial stiffening – in reduction of nitric oxide activity, promotion of adhesion molecules, expression of cytokines, uptake of oxidized LDL cholesterol and generation of foam cells, production of extracellular matrix protein (collagen and elastin) and formation of cross-links [Citation15]. It was shown that significance of sRAGE plasma levels in cardiovascular and metabolic diseases differ in diabetic and non-diabetic subjects. Falcone et al. found significantly lower sRAGE in patients with angiografically documented coronary artery disease in nondiabetic men [Citation16]. Our group reported that decreased sRAGE concentrations were independently associated with increased aortic stiffness but only in nondiabetic hypertensive subjects from a general population [Citation15]. In patients with type 2 diabetes, however, the relationship can be quite different. Indeed, several authors observed that elevated sRAGE levels were associated with the increased risk of CHD in type 2 diabetes [Citation28–31]. Not only in case of diabetes but also in case of renal dysfunction there is a controversy. The serum levels of sRAGE are also elevated in patients with impaired renal function. Kalousova et al. showed that patients with renal insufficiency have increased sRAGE levels in independent of the cause of kidney disease [Citation32]. Therefore, some authors have proposed that the serum levels of sRAGE should be used in conjunction with the serum levels of AGEs [Citation33–35]. The AGEs/sRAGE ratio might be a better biomarker of oxidative stress than sRAGE alone. If only low sRAGE status is considered as a disease biomarker it cannot be applicable to diabetes and renal insufficiency. We did not perform the measurement of AGEs levels in our study, however it should be pointed out that our whole cohort consisted of nondiabetic subjects and all respondents had renal parameters in the normal reference range.
The role of sRAGE is complex. It may be considered as an endogenous protective factor against oxidative stress and some authors suggested that it is a possible marker of future chronic disease [Citation33]. On the basis of the aforementioned data, we may suppose that low levels of sRAGE are inversely associated with higher risk of disease in non-diabetic subjects.
Importantly, our finding suggests that offspring probably might have a lower antioxidant protection than controls because the plasma levels of sRAGE are negatively related to effect of oxidative stress. We can just speculate about the cause of low sRAGE levels in these young asymptomatic individuals. The increased cardiovascular risk in the offspring group might be determined not only by hereditary genetic mechanisms but also by acquired lifestyle habits, social status, education and their interaction. This thesis is supported by significantly higher percentage of smokers in our offspring group.
From this point of view, it is interesting to mention that we might influence intake of AGE by diet. It was shown that preparation of common food under varying conditions of water and heat has a different effect on AGE content. Indeed, frying, grilling and roasting generate more AGEs compared to boiling and steaming. Higher consumption of fish, vegetables, fruits and whole grains and lower intake of solid fats, fatty meats and highly processed foods leads to lesser intake of AGEs [Citation35,Citation36]. These findings are consistent with recommendations of several professional organizations for cardiovascular prevention [Citation37].
Another point is whether we have therapeutic possibilities how to beneficially affect AGE/RAGE axis. Miyata et al. demonstrated that the renin-angiotensin system (RAS) blockers -angiotensin converting enzyme inhibitors (ACEis) and angiotensin receptor blockers (ARBs) inhibit the formation of AGEs in Maillard reactions in vitro [Citation38]. Low sRAGE level was associated with significantly higher aPWV only in subjects not treated with RAS blockers in the general population study [Citation15]. Favourable effects of RAS inhibitors are supposed to be in their complex antioxidant, antitrombotic and profibrinolytic activities [Citation39]. Other specific drugs affecting the AGE/RAGE axis such as the crosslinks breakers (alagebrium) or AGE formation inhibitors (aminoguanidin) did not reach clinical utilization [Citation40].
Our results have to be interpreted within the context of its limitations. The present study has a cross sectional nature that means the cause and consequence cannot be answered on the basis of our data. We may only presume on the basis of previous studies that higher aPWV and lower sRAGE levels are associated with higher cardiovascular risk. Second, the examined cohort of offspring was relatively small. From the 648 addressed parents with premature myocardial infarction, only 121 offspring were interested in examination. On the other hand, we may suppose that these individuals were more interested in their health and probably better lifestyle habits. Third, there was a difference in sex proportion (more males among the offspring) but our regression analyses were adjusted for sex. Fourth, the physical activity data were determined only based on the completed questionnaire and we did not test the physical condition in any way. Fifth, smoking is a major cardiovascular risk factor and was shown to influence serum levels of sRAGE. In our sample, offspring reported more frequently history of smoking or current smoking. Therefore, we adjusted regression analyses for this factor as well as we ran sensitivity analyses including only nonsmokers and we obtained confirmatory results. Finally, we did not perform the measurement of AGEs levels to obtain AGEs/sRAGE ratio as a possible universal oxidative stress biomarker. However, in our survey we can use only sRAGE levels alone because our cohort consisted of nondiabetic subjects with normal renal function.
In conclusion, our present study reports for the first time that the offspring of parents with early onset of CHD had significantly lower sRAGE levels suggesting a possible shift in the oxidative balance between stressors and defence mechanisms that may influence a higher cardiovascular risk in the future. The acquired disadvantage of a genetic predisposition together with unhealthy lifestyle habits leads to a higher probability of cardiovascular risk factors, and therefore to a higher risk of cardiovascular disease manifestation. In practice, the overall objective should be to motivate these predisposed individuals to improve their modifiable CV risk factors related to a healthy diet, avoidance of tobacco and taking regular exercise. The measurement of sRAGE and/or AGEs/sRAGE ratio might be a valuable predictor for identifying and stratification of cardiovascular risk. Further investigation is needed to give a complex understanding of sRAGEs and its role in prevention of cardiovascular diseases.
Supplementary_materials.docx
Download MS Word (22.3 KB)Disclosure statement
None of the authors has a conflict of interest with regard to the data presented in this article.
Additional information
Funding
References
- Murabito JM, Pencina MJ, Nam BH, et al. Sibling cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults. JAMA. 2005;294(24):3117–3123.
- Nasir K, Budoff MJ, Wong ND, et al. Family history of premature coronary heart disease and coronary artery calcification: multi-ethnic study of atherosclerosis (MESA). Circulation. 2007;116(6):619–626.
- Lloyd-Jones DM, Nam BH, D’Agostino RB, et al. Parental cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults: a prospective study of parents and offspring. JAMA. 2004;291(18):2204–2211.
- Goldin A, Beckman JA, Schmidt AM, et al. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114(6):597–605.
- Kalousova M, Zima T, Tesař V, at al. Advanced glycoxidation end products in chronic diseases-clinical chemistry and genetic background. Mutat Res. 2005;579(1/2):37–46.
- Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–1625.
- Makita Z, Radoff S, Rayfield EJ, et al. Advanced glycosylation end products in patients with diabetic nephropathy. N Engl J Med. 1991;352(12):836–842.
- Salahuddin P, Rabbani G, Khan RH. The role of advanced glycation end products in various types of neurodegenerative disease: a therapeutic approach. Cell Mol Biol Lett. 2014;19(3):407–437.
- Basta G. Receptor for advanced glycation endproducts and atherosclerosis: from basis mechanisms to clinical implication. Atherosclerosis. 2008;196(1):9–21.
- Koyama H, Yamamoto H, Nishizawa Y. RAGE and soluble RAGE: potential therapeutic targets for cardiovascular disease. Mol med. 2007;13(11/12):625–635.
- Park L, Raman KG, Lee KJ, et al. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation end-products. Nat Med. 1998;4(9):1025–1031.
- Yamagishi S, Matsui T. Soluble form of a receptor for advanced glycation end products (sRAGE) as a biomarker. Front Biosci. 2010;2:1184–1195.
- Yan SF, D´Agati V, Schmidt AM. Receptor for advanced glycation endproducts (RAGE): a formidable force in the pathogenesis of the cardiovascular complications of diabetes and aging. Curr Mol Med. 2007;7:699–710.
- Geroldi D, Falcone C, Emanuele E, et al. Decreased plasma levels of soluble receptor for advanced glycation end-products in patients with essential hypertension. J Hypertens. 2005;23(9):1725–1729.
- Mayer O, Seidlerová J, Filipovský J, et al. Soluble receptor for advanced glycation end products and increased aortic stiffness in the general population. Hypertens Res. 2016;39(4):266–271.
- Falcone C, Emanuele E, D´Angelo A, et al. Plasma levels of soluble receptor for advanced glycation end products and coronary artery disease in nondiabetic men. ATVB. 2005;25(5):1032–1037.
- Cífková R, Škodová Z, Lánská V, et al. Prevalence, awareness, treatment, and control of hypertension in the Czech Republic. Results of two nationwide cross-sectional surveys in 1997/1998 and 2000/2001, Czech Post-MONICA study. J Hum Hypertens. 2004;18(8):571–579.
- Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American heart association/national heart, lung, and blood institute scientific statement. Circulation. 2005;112(17):2735–2752.
- Wohlfahrt P, Krajčoviechová A, Seidlerová J, et al. Lower-extremity arterial stiffness vs. aortic stiffness in the general population. Hypertens Res. 2013;36(8):718–724.
- Van Bortel LM, Laurent S, Boutouyrie P, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30(3):445–448.
- Wilkinson IB, Fuchs SA, Jansen IM, et al. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens. 1998;16(Suppl):2079–2084.
- Prasad K, Dhar I, Caspar-Bell G. Role of advanced glycation end products and its receptors in the pathogenesis of cigarette smoke-induced cardiovascular disease. Int J Angiol. 2015;24:75–80.
- Stern DM, Yan SD, Yan SF, et al. Receptor for advanced glycation endproducts (RAGE) and the complications of diabetes. Ageing Res Rev. 2002;1(1):1–15.
- Schmidt AM, Hasu M, Popov D, et al. Receptor for advanced glycation endproducts (AGEs) has a central role in vessel wall interactions and gene activation in response to circulating AGE proteins. Proc Natl Acad Sci USA. 1994;91(19):8807–8811.
- Mahajan N, Malik N, Bahl A, et al. Correlation among soluble markers and severity of disease in non-diabetic subjects with pre-mature coronary artery disease. Mol Cell Biochem. 2009;330(1/2):201–209.
- Cohen R, Budoff M, McClelland RL, et al. Significance of a positive family history for coronary heart disease in patients with a zero coronary artery calcium score (from the multi-ethnic study of atherosclerosis). Am J Cardiol. 2014;114(8):1210–1214.
- Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588–2605.
- Tan KC, Shiu SW, Chow WS, et al. Association between serum levels of soluble receptor for advanced glycation end products in type 2 diabetes. Diabetologia. 2006;49(11):2756–2762.
- Nakamura K, Yamagishi SI, Adachi H, et al. Elevation of soluble form of receptor for advanced glycation end products (sRAGE) in diabetic subjects with coronary artery disease. Diabetes Metab Res Rev. 2007;23(5):368–371.
- Colhoun HM, Betteridge DJ, Durrington P, et al. Total soluble and endogenous secretory receptor for advanced glycation end products as predictive biomarkers of coronary heart disease risk in patients with type 2 diabetes: an analysis from the CARDS trial. Diabetes. 2011;60(9):2379–2385.
- Fujisawa K, Katakami N, Kaneto H, et al. Circulating soluble RAGE as a predictive biomarker of cardiovascular event risk in patients with type 2 diabetes. Atherosclerosis. 2013;227(2):425–458.
- Kalousová M, Hodková M, Kazderová M, et al. Soluble receptor for advanced glycation end products in patients with decreased renal function. Am J Kidney Dis. 2006;47(3):406–411.
- Prasad K. Low levels of serum soluble receptors for advanced glycation end products, biomarkers for disease state: myth or reality. Int J Angiol. 2014;23(1):11–16.
- Corica D, Aversa T, Ruggeri RM, et al. Could AGE/RAGE-related oxidative homeostasis dysregulation enhance susceptibility to pathogenesis of cardio-metabolic complications in childhood obesity? Front Endocrinol. 2019;10:426.
- Prasad K, Mishra M. AGE–RAGE stress, stressors, and antistressors in health and disease. Int J Angiol. 2018;27:1–12.
- Uribarri J, Woodruff S, Goodman S, et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. 2010;110(6):911–916.e12.
- Arnett DK, Blumenthal RS, Albert MA, et al. ACC/AHA guideline on the primary prevention of cardiovascular disease. Circulation. 2019;17:CIR0000000000000678.
- Miyata T, van Ypersele de Strihou C, Ueda Y, et al. Angiotensin II receptor antagonists and angiotensin-converting enzyme inhibitors lower in vitro the formation of advanced glycation end products: biochemical mechanisms. J Am Soc Nephrol. 2002;13(10):2478–2487.
- Montecucco F, Pende A, Mach F. The renin-angiotensin system modulates inflammatory processes in atherosclerosis: evidence from basic research and clinical studies. Mediators Inflamm. 2009;2009:752406.
- Brownlee M, Vlassara H, Kooney A, et al. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science. 1986;232(4758):1629–1632.
