Abstract
Purposes: Many studies have indicated that orthostatic hypotension (OH) may be a risk factor for dementia and stroke, but the results have been inconsistent. To further ascertain the links between OH and cognition or stroke, a meta-analysis was performed.
Methods: The Chinese Biomedical Database, PubMed, Web of Science, and the Cochrane Library database were searched (up to March 2019) to identify prospective cohort studies that examined the associations between OH and the risks of stroke and dementia among adult populations. Subgroup analyses and meta-regression analyses were conducted to identify sources of heterogeneity. We also performed Begg’s test and Egger’s test to assess publication bias.
Results: A total of 3490 articles were identified, and 18 prospective observational cohort studies were ultimately included. Among these studies, eight prospective studies were about stroke, nine studies were about cognition and one study reported data about both stroke and dementia. Meta-analysis revealed an association between OH and worse cognition (hazard ratio (HR): 1.18, 95% confidence interval (CI): 1.03–1.35, I2 = 69.5%). For dementia, the pooled HR was 1.30, with 95% CI: 1.14–1.48, I2 = 31.0%. In addition, we found that OH was associated with a higher risk of stroke (HR: 1.36, 95% CI = 1.17–1.57, I2 = 67.3%). No publication bias was detected.
Conclusion: This meta-analysis provides evidence that OH was associated with worse cognition. OH accounted for a 30% increase in the risk of dementia and a 36% increase in the risk of stroke.
Introduction
Orthostatic hypotension (OH) is a condition in which the blood pressure drops in standing position, with estimated prevalence varied from 6% to 81% in community-dwelling older people [Citation1]. Traditionally, OH has been defined as a sustained reduction in systolic blood pressure (BP) of at least 20 mmHg and/or diastolic BP decreasing by at least 10 mmHg for the first 3 min of standing or head tilting at least 60° [Citation2]. Among supine hypertensive patients, a systolic BP decrease of at least 30 mmHg may be more appropriate for OH diagnosis [Citation3,Citation4]. OH is common, but it is a significant burden for healthcare systems and a major public health challenge. Several studies have suggested associations between OH and the increased risk of cardiovascular diseases, falls, and mortality [Citation5–7], as well as associations with diseases of brain hypoperfusion, such as stroke [Citation8] and cognitive functioning [Citation9,Citation10]. However, the evidence for associations between OH and strokes and cognitive dysfunction is still contradictory. Therefore, we conducted a literature search for the latest evidence of the relationship between OH and cognition and stroke.
Methods
Materials and methods
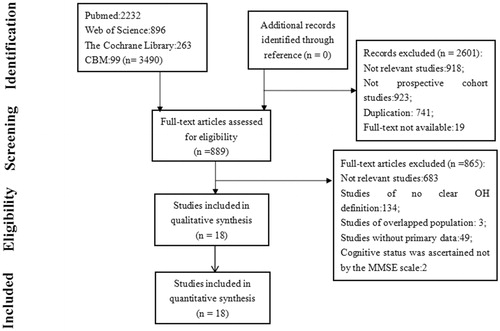
This systematic review was developed following the PRISMA guidelines. The Chinese Biomedical Database (CBM), PubMed, Web of Science, and the Cochrane Library database were systematically searched from January 1996, when the consensus definition of OH was established, to March 2019. Search keywords were as follows: (orthostatic hypotension OR postural hypotension OR orthostasis OR orthostatic blood pressure) combined with (stroke OR dementia OR cognitive decline OR cognitive impairment OR Alzheimer's disease OR vascular dementia). The language was restricted to English and Chinese. To maximize the search for potentially eligible studies, we also reviewed the reference lists of previous systematic reviews and relevant articles manually. Two authors (Min Min and Tingting Shi) independently screened all titles and abstracts first; then they obtained the full text articles and further screened. Disagreements were resolved by consensus.
Study selection
Studies were eligible if the following criteria were met: (1) full text manuscript was published in a peer-reviewed journal; (2) prospective cohort studies; (3) participants were of mean or median age ≥18 years; and (4) diagnosis of OH was equivalent to the 1996 consensus.
Exclusion criteria: (1) when the study population was partially overlapping among studies, only the study with the largest sample size was included; (2) studies where the adapted hazard risks (HRs)/adapted relative risks (RRs)/adapted odd risks (ORs) of developing dementia/cognitive decline/stroke after follow-up were not reported for participants with and without baseline OH were excluded.
Data extraction
Outcomes were expressed as adjusted RRs/ORs/HRs with 95% confidence interval (CI) and the main characteristics of the included studies, such as study design, country, sample size, duration of follow-up, diagnosis criteria, and prevalence of OH were independently extracted from the original studies by two reviewers (Min Min and Tingting Shi). Disagreements were resolved by discussion to consensus.
Quality assessment
Two independent investigators (Min Min and Tingting Shi) evaluated the risk of bias in all included studies using the Newcastle-Ottawa scale (NOS). This scale assesses the study quality from three aspects: selection, comparability, and ascertainment of outcomes [Citation11]. NOS scores ranging from 0 to 3, 4 to 6, and 7 to 9 points implied studies of low, moderate, and high quality, respectively. Disagreements were resolved by discussion to consensus.
Data synthesis and statistical analysis
We performed all statistical analyses in Stata version 14. Summary estimates were obtained using the Mantel–Haenszel method. Statistical heterogeneity was quantified using the I2 statistic [Citation12]. Conventionally, an I2 value below 25% is thought to indicate low heterogeneity. When the I2 value is between 25% and 75%, it represents moderate heterogeneity. When above 75%, it is considered high heterogeneity. Because random effects are thought to be more conservative, we adopted the random-effects model to quantify the pooled estimates in our meta-analysis. Publication bias was assessed qualitatively by inspecting the symmetry of the funnel plots and quantitatively by Egger’s test and Begg’s test [Citation13,Citation14]. Furthermore, we also conducted a meta-regression analysis to test the influences of study-level covariables. We further performed a sensitivity analysis by sequentially excluding one study at a time to assess the robustness of the aggregated results.
Results
Characteristics of studies included
Of the identified 3490 articles, 18, comprising 151,677 individuals, who were initially free of dementia or stroke, were included in this systematic review. Among these 18 articles, one study reported data about both cognition and stroke [Citation15]. Eight studies were only about the relationship between OH and cognition [Citation15–23], and nine were only concerning the association between OH and stroke [Citation15,Citation24–32]. The main characteristics of the 18 prospective studies are presented in . The results of the study quality assessment are shown in Table S1. The study flow chart for the selection process is shown in .
Table 1. Characteristics of the included studies.
Table 2. Characteristics of studies included in the meta-analysis.
Meta-analysis for the association between OH and cognition
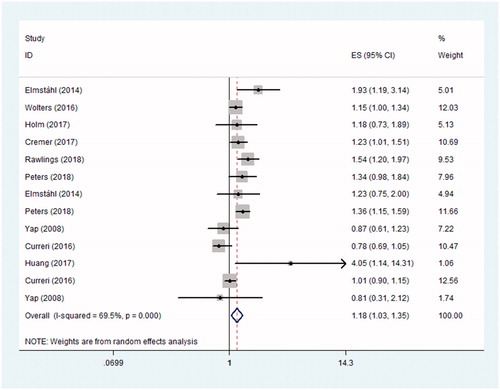
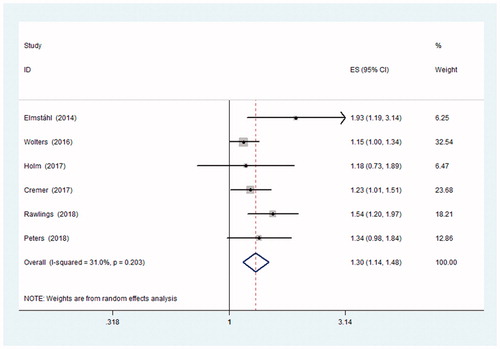
Nine studies that reported cognitive outcomes, including cognitive decline (CD), cognitive impairment (CI), and dementia [Citation15–23]. Six studies, consisting of 48,179 participants, referred to dementia [Citation15–20]. The diagnostic criteria were various among the included studies. One study ascertained dementia based on ICD-9 [Citation15], two other studies by DSM-III-R [Citation17,Citation18], and the remaining three studies diagnosed dementia based on DSM-IV [Citation16,Citation19,Citation20]. Five studies that included a total of 5829 participants reported data concerning CI [Citation16,Citation21–23]. Of the five CI studies, CI was defined as MMSE ≤23 in one study [Citation21], as a score of 0 or 1 on the three words delayed recall task of the MMSE in another [Citation16], and as MMSE ≤24 in the remaining three [Citation20,Citation22,Citation23]. Additionally, two studies that included 2845 participants also reported results about CD [Citation21,Citation23]. One study [Citation21] defined CD as a drop in MMSE score of ≥1 point, and the other study [Citation23] defined CD as a MMSE score drop of ≥3 points.
The results of the included studies were mixed. Some studies suggested a positive effect, whereas others indicated null associations. The pooled results revealed a significant association between OH and dementia. When dementia, CD and CI were pooled together, the aggregated HR was 1.18 with 95% CI: 1.03–1.35, I2 = 69.5%, indicating that OH was associated with worse cognition. Participants with OH were significantly more likely to have dementia than those without OH (adjusted pooled HR = 1.30; 95% CI: 1.143–1.48; I2=31%) ( and and ).
Table 3. Summary of effect measure (HR and 95% CI).
Subgroup analysis for cognition
When the studies were grouped by the age of the participants, elderly people with OH were more likely to have dementia (≥65 years: adjusted HR = 1.26, 95% CI 1.09–1.46, n = 4; <65 years: adjusted HR = 1.27, 95% CI 0.97–1.65, n = 3) (Figure S1). When stratified by hypertension status, OH was likely to increase the risk of developing dementia among hypertensive participants (hypertensive: adjusted HR = 1.39, 95% CI 1.02–1.88, I2 = 75.1%, n = 3; nonhypertensive: adjusted HR = 1.21, 95% CI 0.96–1.52, I2 = 0, n = 2). Furthermore, OH seemed to be related to a high risk of dementia among participants with diabetes (adjusted HR = 1.81, 95% CI 1.07–3.07, n = 2) but not for subjects without diabetes (adjusted HR = 1.20, 95% CI 0.995–1.45, n = 2). In addition, no significant difference was found between groups by the different method of dementia ascertainment (Figure S2).
Relationship between OH and stroke
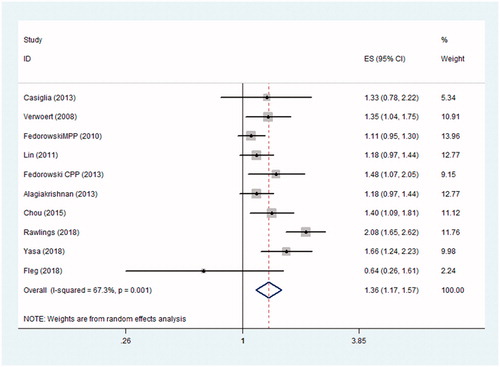
In total, we identified 10 prospective studies involving 112,338 participants that addressed the associations between OH and stroke [Citation15,Citation24–32]. Four of these studies revealed that OH was associated with the risk of stroke [Citation15,Citation28,Citation30,Citation31]. The remaining six studies failed to obtain a statistically significant association between OH and stroke after adjustment for confounding factors [Citation24–27,Citation29,Citation32]. The pooled analysis showed a significant association between OH and stroke (adjusted pooled HR= 1.38, 95% CI: 1.19–1.59, I2= 68.1%, n = 9) (). The forest plots are shown in .
Subgroup analysis for stroke
Only two studies addressed the association between OH and the risk of ischaemic stroke [Citation15,Citation30]. The pooled estimate for the risk of developing ischaemic stroke was 1.71 with 95% CI: 1.17–2.52, n = 2. According to the age of the participants, the risk of stroke was increased among younger subjects with OH (<65 years: adjusted HR = 1.38, 95% CI 1.14–1.67, n = 7) but not for elderly people (≥65 years: adjusted HR = 1.17, 95% CI 0.98–1.39, n = 5; Figure S3). The meta-regression analysis suggested that factors such as age, gender, BMI, the prevalence of OH, and follow-up duration had little apparent effect on the links between OH and stroke (Table S2).
Sensitivity analyses
A sensitivity analysis was conducted by deleting studies sequentially. The results did not find any single study that significantly affected the pooled estimates, supporting the robustness of the evidence (Tables S3 and S4, and Figure S4).
Publication bias
By visual inspections of the funnel plots and calculation of the bias coefficient of Egger’s test and Begg’s test, no evidence of publication bias was found for the associations between OH and worse cognition (Begg's test: p = .86; Egger's test: p = .26), dementia (Begg's test: p = .45; Egger's test: p = .08), and stroke events (Begg's test: p = .25; Egger's test:p = .27) (Figures S5, S6, and S7).
Discussion
In this meta-analysis, we provided evidence of strong links between OH and both worse cognition and stroke. Our findings were consistent with the results of previous systematic reviews [Citation5,Citation33,Citation34]. Compared with the recently published study [Citation34], in which OH was found to be associated with a decreased cognitive performance score, our approach was different from theirs. The authors of the previous study extracted the mean and standard deviation of the decreased cognition score, and we extracted the risk ratio. As expected, hypertensive participants with OH had higher risks for developing dementia, in line with the results reported in one recent study [Citation35]. Studies have indicated that both hypertension [Citation36] and OH [Citation37] are associated with impaired baroreflex. Coexistent OH and hypertension represent a more severe baroreflex dysfunction and exaggerated blood pressure variability. In addition, among elderly people, there was a significant relationship between OH and dementia. For stroke, a statistically significant link was observed among participants younger than 65 years.
There are several pathophysiological mechanisms involving the association between OH, dementia, and stroke. Cerebral hypoperfusion has been initially recognized as an important mediator for the links between OH and worse cognition and stroke [Citation8]. Studies have suggested that cerebral hypoperfusion may lead to regional structural changes such as leukoaraiosis and grey matter atrophy, which underly the neurodegeneration process in dementia [Citation38,Citation39]. However, other studies indicated that the associations between lower orthostatic blood pressure and cognitive status were not mediated by white matter hyperintensities (WMH) and grey matter atrophy [Citation40,Citation41]. Moreover, baroreflex dysfunction, a marker of autonomic nervous system imbalance, was also suggested to be implicated in the pathogenesis of the association between OH and neurological complications [Citation42]. The compensation mechanism for decreased cerebral perfusion pressure is not sufficient to maintain adequate cerebral blood flow, eventually leading to ischaemic brain damage [Citation43]. The links between OH and worse cognitive function were indicated by dysregulation of cerebral blood flow in patients with OH [Citation44,Citation45]. A recent cross-sectional study concluded that OH in elderly people, characterized by cerebral damage or dysregulation of cerebral blood flow, was not related to cognitive function [Citation46]. Nevertheless, there was a study that suggested that autonomic dysfunction is a marker of neurodegenerative diseases rather than a causative factor [Citation47]. Cerebral autoregulation may be attenuated with ageing [Citation48]. Diabetes, hypertension and other complications may impair or override cerebral autoregulation, which is associated with both OH and increased cognitive disorder or incident stroke [Citation49,Citation50]. Though these complications may introduce false associations, most of them were adjusted in the individual studies. To date, relevant pathophysiological mechanisms are multiple but inconsistent among studies; therefore, more studies are warranted in the future.
Strengths and limitations
Compared with previous findings, our study included more and newer articles; it provided statistically significant and robust evidence that OH was associated with worse cognition and was related to a high risk of dementia and stroke. Moreover, this study discussed the links between OH and neurological complications (worse cognition and stroke) among different populations stratified by age and in a hypertensive population. However, as the adjusted factors were inconsistent between the included studies, some confounding factors, such as demographics and disease status, cannot be completely excluded. The measurement protocols, definitions of OH and diagnostic criteria for the events (cognitive decline, dementia, and stroke) varied across the included studies, which increased the heterogeneity. Furthermore, the small number of individual studies was insufficient to further analyze the relationship between postural hypotension and subtypes of stroke. Finally, due to the lack of relevant clinical trials, our review only comprised observational studies, which hindered the power to make any causal inferences.
Conclusion
This systematic review and meta-analysis showed that OH may be associated with worse cognition and stroke. OH is a common condition and is easy to measure; thus, it may help to identify subjects with a higher risk of dementia and stroke. Future research should address the effect of OH interventions to potentially decrease the risk of dementia and stroke.
Figure_S7.tif
Download TIFF Image (236.4 KB)Figure_S6.tif
Download TIFF Image (241 KB)Figure_S5.tif
Download TIFF Image (1.5 MB)Figure_S4.docx
Download MS Word (32.9 KB)Figure_S3.tif
Download TIFF Image (699.2 KB)Figure_S2.tif
Download TIFF Image (1.3 MB)Figure_S1.tif
Download TIFF Image (446.2 KB)Table_S4.docx
Download MS Word (15 KB)Table_S3.doc
Download MS Word (64.5 KB)Table_S2.docx
Download MS Word (12.8 KB)Table_S1.docx
Download MS Word (16.2 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Saedon NI, Tan MP. The prevalence of orthostatic hypotension: a systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci. 2019. doi:10.1093/gerona/gly188.
- Kaufmann H. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Clin Auton Res. 1996;6(2):125–126.
- Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21(2):69–72.
- Gibbons CH, Schmidt P, Biaggioni I, et al. The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. J Neurol. 2017;264(8):1567–1582.
- Ricci F, Fedorowski A, Radico F, et al. Cardiovascular morbidity and mortality related to orthostatic hypotension: a meta-analysis of prospective observational studies. Eur Heart J. 2015;36(25):1609–1617.
- Hartog LC, Schrijnders D, Landman GWD, et al. Is orthostatic hypotension related to falling? A meta-analysis of individual patient data of prospective observational studies. Age Ageing. 2017;46(4):568–575.
- Angelousi A, Girerd N, Benetos A, et al. Association between orthostatic hypotension and cardiovascular risk, cerebrovascular risk, cognitive decline and falls as well as overall mortality: a systematic review and meta-analysis. J Hypertens. 2014;32(8):1562–1571.
- Eigenbrodt ML, Rose KM, Couper DJ, et al. Orthostatic hypotension as a risk factor for stroke: the atherosclerosis risk in communities (ARIC) study, 1987–1996. Stroke. 2000;31(10):2307–2313.
- Sambati L, Calandra-Buonaura G, Poda R, et al. Orthostatic hypotension and cognitive impairment: a dangerous association? Neurol Sci. 2014; 35(6):951–957.
- Udow SJ, Robertson AD, MacIntosh BJ, et al. Under pressure: Is there a link between orthostatic hypotension and cognitive impairment in -synucleinopathies? J Neurol Neurosurg Psychiatr. 2016;87(12):1311–1321.
- Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses;Ottawa (ON): Ottawa Hospital Research Institute. 2014. Availble at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]: Cochrane Collaboration; 2011. Available: http://handbook-5-1.cochrane.org/
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101.
- Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634.
- Rawlings AM, Juraschek SP, Heiss G, et al. Association of orthostatic hypotension with incident dementia, stroke, and cognitive decline. Neurology. 2018;91(8):e759–e768.
- Elmståhl S, Widerström E. Orthostatic intolerance predicts mild cognitive impairment: incidence of mild cognitive impairment and dementia from the Swedish general population cohort Good Aging in Skåne. Clin Interv Aging. 2014;9:1993–2002.
- Wolters FJ, Mattace-Raso FUS, Koudstaal PJ, et al. Orthostatic hypotension and the long-term risk of dementia: a population-based study. PLoS Med. 2016;13(10):e1002143.
- Holm H, Nägga K, Nilsson ED, et al. Longitudinal and postural changes of blood pressure predict dementia: the Malmö Preventive Project. Eur J Epidemiol. 2017;32(4):327–336.
- Cremer A, Soumaré A, Berr C, et al. Orthostatic hypotension and risk of incident dementia: results from a 12-year follow-up of the three-city study cohort. Hypertension. 2017;70(1):44–49.
- Peters R, Anstey KJ, Booth A, et al. Orthostatic hypotension and symptomatic subclinical orthostatic hypotension increase risk of cognitive impairment: an integrated evidence review and analysis of a large older adult hypertensive cohort. Eur Heart J. 2018;39(33):3135–3143.
- Yap PL, Niti M, Yap KB, et al. Orthostatic hypotension, hypotension and cognitive status: early comorbid markers of primary dementia? Dement Geriatr Cogn Disord. 2008;26(3):239–246.
- Huang H, Zheng T, Liu F, et al. Orthostatic hypotension predicts cognitive impairment in the elderly: findings from a cohort study. Front Neurol. 2017;8:121.
- Curreri C, Giantin V, Veronese N, et al. Orthostatic changes in blood pressure and cognitive status in the elderly: the Progetto Veneto Anziani study. Hypertension. 2016;68(2):427–435.
- Casiglia E, Tikhonoff V, Caffi S, et al. Orthostatic hypotension does not increase cardiovascular risk in the elderly at a population level. Am J Hypertens. 2014;27(1):81–88.
- Verwoert GC, Mattace-Raso FU, Hofman A, et al. Orthostatic hypotension and risk of cardiovascular disease in elderly people: the Rotterdam study. J Am Geriatr Soc. 2008;56(10):1816–1820.
- Fedorowski A, Stavenow L, Hedblad B, et al. Orthostatic hypotension predicts all-cause mortality and coronary events in middle-aged individuals (The Malmo Preventive Project). Eur Heart J. 2010;31(1):85–91.
- Lin ZQ, Xie ZQ, Wu ZQ, et al. [Correlation between orthostatic hypotension and cardiovascular risks in elderly population]. Zhonghua Yi Xue Za Zhi. 2011;91:2530–2533.
- Fedorowski A, Wahlstrand B, Hedner T, et al. Systolic and diastolic component of orthostatic hypotension and cardiovascular events in hypertensive patients: the Captopril Prevention Project. J Hypertens. 2014;32(1):75–81.
- Alagiakrishnan K, Patel K, Desai RV, et al. Orthostatic hypotension and incident heart failure in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2014;69:223–230.
- Chou RH, Liu CJ, Chao TF, et al. Association between orthostatic hypotension, mortality, and cardiovascular disease in Asians. Int J Cardiol. 2015;195:40–44.
- Yasa E, Ricci F, Magnusson M, et al. Cardiovascular risk after hospitalisation for unexplained syncope and orthostatic hypotension. Heart. 2018;104(6):487–493.
- Fleg JL, Evans GW, Margolis KL, et al. Orthostatic hypotension in the ACCORD (action to control cardiovascular risk in diabetes) blood pressure trial: prevalence, incidence, and prognostic significance. Hypertension. 2016;68(4):888–895.
- Min M, Shi T, Sun C, et al. The association between orthostatic hypotension and dementia: a meta-analysis of prospective cohort studies. Int J Geriatr Psychiatry. 2018;33(12):1541–1547.
- Iseli R, Nguyen VTV, Sharmin S, et al. Orthostatic hypotension and cognition in older adults: a systematic review and meta-analysis. Exp Gerontol. 2019;120:40–49.
- McNicholas T, Tobin K, Carey D, et al. Is baseline orthostatic hypotension associated with a decline in global cognitive performance at 4-year follow-up? Data from TILDA (The Irish Longitudinal Study on Ageing). J Am Heart Assoc. 2018;7(19):e008976.
- Mancia G, Grassi G. The autonomic nervous system and hypertension. Circ Res. 2014;114(11):1804–1814.
- Goldstein DS, Pechnik S, Holmes C, et al. Association between supine hypertension and orthostatic hypotension in autonomic failure. Hypertension. 2003;42(2):136–142.
- Ballard C, O'Brien J, Barber BOB, et al. Neurocardiovascular instability, hypotensive episodes, and MRI lesions in neurodegenerative dementia. Annals NY Acad Sci. 2000;903:442–445.
- Suter OC, Sunthorn T, Kraftsik R, et al. Cerebral hypoperfusion generates cortical watershed microinfarcts in Alzheimer disease. Stroke. 2002;33(8):1986–1992.
- O'Hare C, Kenny RA, Aizenstein H, et al. Cognitive status, gray matter atrophy, and lower orthostatic blood pressure in older adults. J Alzheimers Dis. 2017;57:1239–1250.
- Suemoto CK, Baena CP, Mill JG, et al. Orthostatic hypotension and cognitive function: cross-sectional results from the ELSA-Brasil study. J Gerontol A Biol Sci Med Sci. 2018;15;74(3):358–365.
- Low PA, Tomalia VA. Orthostatic hypotension: mechanisms, causes, management. J Clin Neurol. 2015;11(3):220–226.
- Novak V, Novak P, Spies JM, et al. Autoregulation of cerebral blood flow in orthostatic hypotension. Stroke. 1998;29(1):104–111.
- Biogeau J, Desmidt T, Dujardin PA, et al. Ultrasound tissue pulsatility imaging suggests impairment in global brain pulsatility and small vessels in elderly patients with orthostatic hypotension. J Stroke Cerebrovasc Dis. 2017;26(2):246–251.
- Centi J, Freeman R, Gibbons CH, et al. Effects of orthostatic hypotension on cognition in Parkinson disease. Neurology. 2017;88(1):17–24.
- Foster-Dingley JC, Moonen JEF, de Ruijter W, et al. Orthostatic hypotension in older persons is not associated with cognitive functioning, features of cerebral damage or cerebral blood flow. J Hypertens. 2018;36(5):1201–1206.
- Allcock LM, Kenny RA, Mosimann UP, et al. Orthostatic hypotension in Parkinson's disease: association with cognitive decline? Int J Geriat Psychiatry. 2006;21(8):778–783.
- Moretti R, Torre P, Antonello RM, et al. Risk factors for vascular dementia: hypotension as a key point. Vasc Health Risk Manag. 2008; 4:395–402.
- Vianna LC, Deo SH, Jensen AK, et al. Impaired dynamic cerebral autoregulation at rest and during isometric exercise in type 2 diabetes patients. Am J Physiol Heart Circ Physiol. 2015;308(7):H681–H687.
- Hughes TM, Sink KM. Hypertension and its role in cognitive function: current evidence and challenges for the future. AJHYPE. 2016;29(2):149–157.