Abstract
Purpose: For primary health care (PHC), hypertension is the number one diagnosis for planned health care visits. The treatment of high blood pressure (BP) and its consequences constitutes a substantial economic burden. In spite of efficient antihypertensive medications, a low percentage of patients reach a well-controlled BP. The PERson-centredness in Hypertension management using Information Technology (PERHIT) Study is a multicentre randomised controlled trial. PERHIT is designed to evaluate the effect of supporting self-management on systolic blood pressure by the use of information technology in Swedish primary health care.
Materials and Methods: After inclusion, 900 patients from 36 PHC centres are randomised to two groups. In the intervention group, patients are provided with a self-management support system including a home-BP monitor and further requested to perform self-reports and measure BP every evening for eight consecutive weeks. In the control group, patients receive treatment as usual.
Results: The primary outcome will be the change in systolic blood pressure in patients with hypertension. In addition, person-centredness, daily life activities, awareness of risk and health care costs will also be evaluated.
Conclusion: The results of this randomised controlled trial with assessment of blood pressure and same-day self-reports will provide patients a tool to understand the interplay between blood pressure and lifestyle applicable to primary health care. The self-management support system may be of importance for improved adherence to treatment and persistence to treatment recommendations.
Introduction
Despite the wide availability of efficient antihypertensive medications, a low percentage of patients reach a well-controlled blood pressure (BP). One explanation for this is low adherence to anti-hypertensive treatment. Even if some improvement in treatment and control of hypertension have been reported with attainment of target BP (<140/90 mmHg) of 37% among patients in Swedish primary health care, these results still urge for improved BP control [Citation1]. Efforts are clearly needed for a larger proportion of the population with hypertension to reach their goal BP. Resistance to hypertension guidelines has been observed among prescribers [Citation2] and non-adherence among patients [Citation3], and these factors become significant barriers to successful hypertension management.
The preventive rather than curative focus of hypertension management makes lifestyle adjustments, as well as medical treatment, an important factor to improve public health and a person-centred perspective may be beneficial [Citation4]. The possibility to assess the patient’s own experience of high blood pressure, effects of medication and lifestyle has not systematically been documented. We believe that self-monitoring has potential to improve motivation and adherence to follow hypertension treatment [Citation5]. Cochrane analyses [Citation6] reported that interventions to improve outcomes of hypertension management may comprise one or several components. The report further concludes that a health-economic evaluation of supplying organised care to patients with hypertension should accompany future studies.
In a systematic review of studies on mobile applications for blood pressure management, it was concluded that positive effects have been shown but there is a need for further randomised controlled trials [Citation7]. In another Cochrane report, the authors concluded that there is a lack of evidence relating to the effects of mobile phone-delivered interventions to increase adherence to medication prescribed for the primary prevention of cardiovascular disease [Citation8].
Increased demand on health care in combination with cost constraints, have set the focus on rethinking health care in order to better fit the purpose of managing chronic conditions on an organisational and individual level [Citation9]. In order to support patients in self-management, health care needs to acknowledge the person with the condition, which in turn implies a need to change old routines and establish new ways of organising and administering care.
In a pilot study, we found that enabling persons with hypertension to monitor and track their BP in relation to medication intake, physical activity, well-being, stress and symptoms could help them to gain understanding of the importance of adherence and persistence to treatment recommendations [Citation10]. There is, however, a need for larger randomised controlled trials to evaluate the effects on BP.
Objectives
Our aim is to lower blood pressure in patients with hypertension in primary care.
Subjects and methods
Trial design
We will conduct a multi-centre randomised controlled trial for twelve months. Randomisation to study groups occurs after completion of baseline assessments. Patients are randomly assigned in a 1:1 ratio to either the intervention group or the control group (). The CONSORT‐EHEALTH checklist is followed. The trial is coordinated from Lund University and Skåne University Hospital with researchers at Lund, Gothenburg and Linköping Universities.
Study setting
Primary health care centres (PHCC) in four Swedish counties (Skåne, Västra Götaland, Östergötland, and Jönköping), surrounding the three participating universities, were recruited as clinical sites. The total number of inhabitants in these regions of Sweden was 3.85 million in 2018 or 38% of the population of Sweden.
Eligibility criteria
Inclusion criteria
Age 18 years or older
Diagnosis of hypertension
On treatment with at least one antihypertensive drug
Understanding of Swedish in order to be able to provide informed consent and to make use of the interactive web-based self-management support system using the mobile phone for answering questions.
Exclusion criteria
Secondary hypertension according to the medical records at the PHCC; terminal illness; pregnancy-induced hypertension; cognitive impairment; impaired vision (not able to read messages on the mobile phone); and psychotic disease.
The intervention
Patients with high blood pressure have been involved in all phases of the design, development and evaluation of the interactive web-based self-management support system that will be used in the intervention group (). The development, validation and results from the piloted version of the system have previously been described in detail [Citation4,Citation10–16]. The system includes four components: (1) a module for self-reporting well-being, symptoms, lifestyle, medication intake and side effects of medication; (2) daily home BP and pulse measurements with a validated BP monitor; (3) tailored weekly motivational messages to encourage lifestyle changes and (4) web-based dashboard to enable patients, as well as physicians and nurses, to examine graphs for visualisation of the patient’s BP in relation to the self-reports. The communication platform for the system, Circadian Questions (CQ), was developed by Circadian Questions AB. CQ is now owned by eSanté AB (http://www.cqmobil.se.).
Figure 2. Overview of the piloted interactive self-management support system. The system consists of: (a) web-based system using the patient’s own mobile phone for the self-report questions, together with optional motivational messages and individualised reminders; (b) BP device; (c) database for real-time registration of the daily self-reports captured from the mobile phone; and (d) web-based platform for real-time visualisation of the patients’ reported data, available after log-in for the patient and physician/nurse, e.g. at consultations [Citation11].
![Figure 2. Overview of the piloted interactive self-management support system. The system consists of: (a) web-based system using the patient’s own mobile phone for the self-report questions, together with optional motivational messages and individualised reminders; (b) BP device; (c) database for real-time registration of the daily self-reports captured from the mobile phone; and (d) web-based platform for real-time visualisation of the patients’ reported data, available after log-in for the patient and physician/nurse, e.g. at consultations [Citation11].](/cms/asset/79c71e24-ddab-431b-ac58-33706e40dd85/iblo_a_1697177_f0002_c.jpg)
A website (www.perhit.se.) for standardisation of procedures and adherence to the study protocol with information, management and monitoring of the study has been developed and is available to patients and healthcare centres. A support function for the study has been established, and site initiation meetings with participating physicians and nurses have been held.
Study procedures
Eligible patients are invited to the PHC for an inclusion visit. A baseline assessment is performed after the informed consent procedure. Patients are thereafter randomised to either the intervention- or the control group.
Patients randomised to the intervention group are given instructions on how to use the system. They are also instructed on how to measure their blood pressure, to log into the database and see their graphs. The nurse or physician tailors the system for the individual needs of each patient by selecting relevant questions regarding drug side effects related to the patient’s drug regimen, choosing motivational messages for a healthy lifestyle (optional) according to the patient’s preferences, and choosing the timing of the daily measurements, self-reports and reminders according to the patient’s wishes. This is in line with the person-centred care that we want to introduce. The patients are provided with a home BP monitor (Microlife BP A6 BT) free of charge. Thereafter they are asked to use the self-management support system and self-report, once daily in the evening, via their own mobile phone for eight weeks. At each occasion, the patients first answer the questions and then measure their BP (mean of three readings) and pulse within the window for answers 5 p.m. – 12 p.m. in the self-report system. The patients are asked to measure their BP at approximately the same time each evening, and these data are self-reported into the mobile phone and after ticking send, they are automatically saved in the database in real time with a time stamp. During the intervention, patients and healthcare professionals have direct access to the self-reported data via the login-restricted web-based platform, i.e. the database. The patients will receive clear instructions on how and who to contact regarding questions about their health or the self-report system and log in to the graph view. The intervention period will be concluded by a follow-up consultation with the responsible nurse or physician. No study intervention will be made in the control group; they will receive treatment as usual. Patients of both groups answer a set of questionnaires at each visit. For both intervention group and control group, there will be three study visits (baseline, after eight weeks, and after one year). Other visits at the PHCC will not be regulated in either group, i.e. they will receive treatment as usual.
Person-centredness, patient participation and engagement
Person-centred care (PCC) [Citation17] is a pragmatic and relational health care model determined and constituted by the participation, needs and capabilities of the patient, and is increasingly implemented in hospitals as well as in primary care in Sweden. In this project, patients are planned to be active partners; a continuous dialogue with patient representatives is maintained throughout the project. Focus group interviews as well as questionnaires will be completed to explore patients’ and professionals’ experience of person-centeredness and a patient-professional partnership.
Outcomes
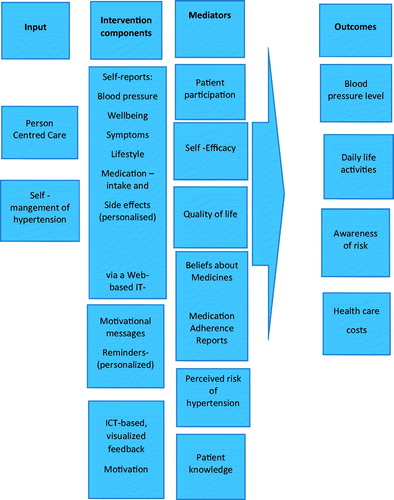
The relationship between intervention components, mediators and outcomes are described in the logic model ().
BP will be measured systematically after 5 min rest in the arm with the participant in a sitting position. We use a validated electronic BP monitor (Microlife BP A6 BT). The mean value of three consecutive measures is displayed and manually recorded in the eCRF. We intend to measure the immediate effects on blood pressure (8 weeks) as well as long-term effects (1 year).
We will also make comparison of groups for achieved BP levels. According to the European guidelines on the management of hypertension [Citation18] we will show final data for the BP goal <140/90 mmHg.
Register data on healthcare resource utilisation one year before baseline and for the full study period will be retrieved for participants in both study groups using county council health-care registers that cover health-care utilisation at all levels of health care (primary care, hospital-based health care) and including health care contacts with physicians, nurses, physiotherapists etc. Information included relates to all health care contacts in primary and hospital based health care as well as inpatient stays. Variables include contact dates (admission, discharge if relevant), main and secondary diagnoses, Diagnosis-Related Group (DRG)-codes, type of caregiver and type of health care contact (visit or phone).
Data from National Prescription Drug Register will be retrieved to assess medication use.
Economic evaluation to support implementation
A model-based analysis of longer-term cost-effectiveness will be performed – provided that the intervention shows patient benefits in terms of a statistically significant lower BP in the intervention group. An economic decision model [Citation19] will be designed and constructed with the aim to simulate lifetime costs and benefits using published long-term data, which are relevant for Sweden, on incidence of clinically relevant complications of hypertension associated with different levels of BP, as well as resource use, costs and patient benefits associated with these complications of hypertension.
In addition, data from the study participants on healthcare costs will be estimated from administrative healthcare databases, including stroke, heart failure and acute myocardial infarction. Likewise, costs of medications will be estimated from the Swedish Prescribed Drug Registry during the study period. Furthermore, the costs of the interactive self-management support system will be estimated using study data taking into consideration the difference between the intervention setting and a real-world application.
Results of the health-economic evaluation will show if the expected patient benefits can meet the demand on healthcare resources of implementation.
Sample size consideration
The power calculation for the planned study is based on t-test for independent groups. A previous study on self-management in the control of hypertension has suggested an average difference in decrease of systolic BP of 5.5 mmHg when comparing self-management and usual care at 12-months follow-up [Citation20]. With an assumed average difference of 5 mmHg, SD = 20 mmHg in both treatment groups (i.e. effect size d = 0.25) and 20% drop out, a sample size of 423 patients per group will, in the proposed study, be required for 90% statistical power, at the 5% significance level.
Recruitment
Recruitment will be conducted in collaboration with the PHC within the four participating healthcare regions. Patients from all PHC regions, private and public, are eligible. We have started the inclusion of study centres and have had meetings with the primary health care organisation. So far, 32 primary health care centres are participating in the study.
Methods
Data collection
Study data were collected and managed using REDCap electronic data capture tools hosted at Clinical Studies Sweden – Forum South, Region Skåne [Citation21,Citation22]. REDCap (Research Electronic Data Capture) is a secure, web-based software platform designed to support data capture for research studies, providing 1) an intuitive interface for validated data capture; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for data integration and interoperability with external sources.
Enrolment; Age, gender, education, ethnicity, duration of hypertension diagnosis, smoking (current, former, never), complications from BP, diabetes, left ventricular hypertrophy (according to the medical records at the PHCC, no extra investigations are made within the study), and antihypertensive medication.
Measurements; BP (systolic and diastolic; mmHg), BMI (weight; kg, height; m)
Questionnaires; Patients’ perceived risk of hypertension, General Self-Efficacy (GSE) Scale (GSE), Quality of life - RAND36, the Beliefs about Medicines Questionnaire (BMQ), Medication Adherence Rating Scale (MARS), and Patients’ Preferences for Patient Participation (4 P).
Laboratory tests; cholesterol, creatinine, cystatin C, HbA1c.
Randomisation
Randomisation to study groups occurs after completion of baseline assessments. To accommodate the number of sites a block randomisation approach was chosen. A statistician not otherwise involved in the project created and uploaded the randomisation table to the randomisation module in REDCap. No members of the current project has access to the randomisation table nor the randomisation module in REDCap.
Data will be monitored at each participating health care centre by an independent and qualified monitor.
Ethical considerations
The study is a multicentre study and has been approved by the Regional Ethical Review Board in Lund, Dnr 2017/311 (May 17, 2017) and Dnr 2019/00036 (January 11, 2019). Ethical considerations include the risk of feeling anxious about one’s BP value when measuring BP at home. It is also possible that other questions will arise when new thoughts and insights materialise, brought on by the use of the system. Another aspect is the risk of feeling supervised or controlled. However, the advantages of gaining control over a situation one previously had a poor understanding of and having the chance to feel empowered are likely to compensate for this. When research with behavioural components is conducted, a common problem is that it is generally the most motivated and health-literate people who are included in a study. This carries the risk that groups who would potentially benefit from an intervention are not included. In order to prevent this risk our intention is to strive for diversity among participants recruited for the study. By including a large number of participants, chances for a diverse study population will increase.
Any change in test results and/or vital signs will be handled according to clinical routine at the primary health care centre.
Registered clinical trial
This trial is registered in ClinicalTrials.gov: NCT03554382.
Discussion
Modern information technology is a tool that might be used in order to improve treatment of hypertension by empowering the patient [Citation14]. In many European countries, a low proportion of patients have well-controlled blood pressure [Citation23]. In a Swedish population of patients with hypertension, about half do not achieve the recommended treatment goals [Citation24]. There are different barriers to optimal hypertension control including factors due to patients and physicians but also the health care system [Citation25].
In the most recent European guidelines (2018), a wider use of out-of-office BP measurement to monitor BP control as well as the importance of evaluating treatment adherence as a major cause of poor BP control are emphasised [Citation26].
In a state-of-the-art re-view the authors assessed the current literature of mHealth [Citation27]. They report two studies of hypertension McManus et al. n = 552 [5] and Magid et al. n = 348 [Citation28]. Together with these large randomised studies we hope to contribute and give evidence of mHealth in care of patients with hypertension.
A systematic review by Mohammadi et al. 2018 asks for necessity to perform further research of Information technology in BP management [Citation7]. Low number of participants and limited intervention period in randomised controlled trials are identified as limitations. They also ask for research not only of hypertensive or of high-risked individuals. Further Kitt et al. 2019 call for assessment and validation of novel technology in hypertension management prior implementation in clinical practice [Citation29]. The system used in our study is compatible with approximately 99% of all mobile phone models used in Sweden; including Java. Almost everyone in Sweden has a mobile phone and it is present almost everywhere all the time. It is easy and quick to perform the self-reports and it is being done to a very high degree. Several didactic means are used in this study. Patients are informed of their target blood pressure at the start of the study and are measuring and reporting their blood pressure themselves on a daily basis. The motivational sms-messages are formed ad modum motivational discussions for inspiration to lifestyle changes; e.g. “Perhaps a nice walk at lunch today?” Patients can log in and follow their blood pressure and hopefully discover possible beneficial correlations to their lifestyle activities. Our piloted study with a person-centered approach, of great importance in self-management, is outlined to give evidence how to go further to improve hypertension care.
Blood pressure control in patients with hypertension attending Swedish primary care has improved but there is still a need for further improvement [Citation1]. One important area is patients’ decline in adherence over time [Citation30]. Use of information technology could be one way to improve the situation by engaging the patient and provide a basis for a more person-centred approach by healthcare personnel [Citation31]. A tool for awareness of BP and daily activities has the potential to make the patient an active partner in care and involved in his/her individual treatment. Understanding of the interplay between BP and daily life activities may increase motivation to adhere to treatment of hypertension and thereby minimise cardiovascular risks. There is, of course, not a single solution or “best” technology design. Better monitoring is one way, use of decision support is another possibility, e.g. utilising non-invasive monitoring of hemodynamic parameters combined with a drug selection algorithm [Citation32].
Acknowledgement
We are indebted to Patrick Reilly for his expertise and advice in editing the manuscript. This work was supported by the Kamprad Foundation under Grant 20170102, the Heart- and Lung Foundation under Grant 20170251, the Swedish Research Council under Grant 2018-02648 and Gothenburg Centre for Person-Centred Care (GPCC).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Holmquist C, Hasselstrom J, Bengtsson BK, et al. Improved treatment and control of hypertension in Swedish primary care: results from the Swedish primary care cardiovascular database. J Hypertens. 2017;35(10):2102–2108.
- Midlöv P, Ekesbo R, Johansson L, et al. Barriers to adherence to hypertension guidelines among GPs in southern Sweden: a survey. Scand J Prim Health Care. 2008;26(3):154–159.
- Alhewiti A. Adherence to long-term therapies and beliefs about medications. Int J Family Med. 2014;2014:479596.
- Hallberg I, Ranerup A, Kjellgren K. Supporting the self-management of hypertension: patients' experiences of using a mobile phone-based system. J Hum Hypertens. 2016;30(2):141–146.
- McManus RJ, Mant J, Haque MS, et al. Effect of self-monitoring and medication self-titration on systolic blood pressure in hypertensive patients at high risk of cardiovascular disease: the TASMIN-SR randomized clinical trial. JAMA. 2014;312(8):799–808.
- Glynn LG, Murphy AW, Smith SM, et al. Interventions used to improve control of blood pressure in patients with hypertension. Cochrane Database Syst Rev. 2010;17(3):CD005182.
- Mohammadi R, Ayatolahi Tafti M, Hoveidamanesh S, et al. Reflection on mobile applications for blood pressure management: a systematic review on potential effects and initiatives. Stud Health Technol Inform. 2018;247:306–310.
- Palmer MJ, Barnard S, Perel P, et al. Mobile phone-based interventions for improving adherence to medication prescribed for the primary prevention of cardiovascular disease in adults. Cochrane Database Syst Rev. 2018;6:CD012675.
- Piepoli MF, Hoes AW, Agewall S, et al. European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR. Eur Heart J. 2016;37(29):2315–2381.
- Taft C, Hallberg I, Bengtsson U, et al. Links between blood pressure and medication intake, well-being, stress, physical activity and symptoms reported via a mobile phone-based self-management support system: a cohort study in primary care. BMJ Open. 2018;8(8):e020849.
- Hallberg I, Taft C, Ranerup A, et al. Phases in development of an interactive mobile phone-based system to support self-management of hypertension. Integr Blood Press Control. 2014;7:19–28.
- Bengtsson U, Kasperowski D, Ring L, et al. Developing an interactive mobile phone self-report system for self-management of hypertension. Part 1: patient and professional perspectives. Blood Press. 2014;23(5):288–295.
- Bengtsson U, Kjellgren K, Hofer S, et al. Developing an interactive mobile phone self-report system for self-management of hypertension. Part 2: content validity and usability. Blood Press. 2014;23(5):296–306.
- Bengtsson U, Kjellgren K, Hallberg I, et al. Improved blood pressure control using an interactive mobile phone support system. J Clin Hypertens. 2016;18(2):101–108.
- Bengtsson U, Kjellgren K, Hallberg I, et al. Patient contributions during primary care consultations for hypertension after self-reporting via a mobile phone self-management support system. Scand J Prim Health Care. 2018;36(1):70–79.
- Hallberg I, Ranerup A, Bengtsson U, et al. Experiences, expectations and challenges of an interactive mobile phone-based system to support self-management of hypertension: patients' and professionals' perspectives. Patient Prefer Adherence. 2018;12:467–476.
- Ekman I, Swedberg K, Taft C, et al. Person-centered care–ready for prime time. Eur J Cardiovasc Nurs. 2011;10(4):248–251.
- Williams B, Mancia G, Spiering W, et al. Practice Guidelines for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. Blood Press. 2018;27(6):314–340.
- Briggs A. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006.
- McManus RJ, Mant J, Bray EP, et al. Telemonitoring and self-management in the control of hypertension (TASMINH2): a randomised controlled trial. Lancet. 2010;376(9736):163–172.
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381.
- Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208.
- Wolf-Maier K, Cooper RS, Kramer H, et al. Hypertension treatment and control in five European countries, Canada, and the United States. Hypertension. 2004;43(1):10–17.
- Odesjo H, Adamsson Eryd S, Franzen S, et al. Visit patterns at primary care centres and individual blood pressure level - a cross-sectional study. Scand J Prim Health Care. 2019;37(1):53–59.
- Ogedegbe G. Barriers to optimal hypertension control. J Clin Hypertens (Greenwich). 2008;10(8):644–646.
- Williams B, Mancia G, Spiering W, et al. ESC/ESH Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36(10):1953–2041.
- Bhavnani SP, Narula J, Sengupta PP. Mobile technology and the digitization of healthcare. Eur Heart J. 2016;37(18):1428–1438.
- Magid DJ, Olson KL, Billups SJ, et al. A pharmacist-led, American Heart Association Heart360 Web-enabled home blood pressure monitoring program. Circ Cardiovasc Qual Outcomes. 2013;6(2):157–163.
- Kitt J, Fox R, Tucker KL, et al. New approaches in hypertension management: a review of current and developing technologies and their potential impact on hypertension care. Curr Hypertens Rep. 2019;21(6):44.
- Holmqvist L, Bostrom KB, Kahan T, et al. Drug adherence in treatment resistant and in controlled hypertension-Results from the Swedish Primary Care Cardiovascular Database (SPCCD). Pharmacoepidemiol Drug Saf. 2018;27(3):315–321.
- Omboni S, Tenti M, Coronetti C. Physician-pharmacist collaborative practice and telehealth may transform hypertension management. J Hum Hypertens. 2019;33(3):177–187.
- Talvik A, Rebora P, Heinpalu-Kuum M, et al. Non-invasive hemodynamic monitoring as a guide to drug treatment of uncontrolled hypertensive patients: effects on home blood pressure in the BEAUTY study. Blood Press. 2018;27(6):368–375.