Abstract
Purpose: The association between serum uric acid (SUA) and pulse wave velocity (PWV), has been extensively evaluated but with some discrepancies in results. A further limitation refers to the fact that only few data were analyzed taking into account the possible effects of gender. The purpose of this study was to estimate the association between SUA and arterial stiffness in general population and hypertensive patients, as a whole population and as divided by gender, by pooling results from existing studies.
Materials and methods: Carotid-femoral and brachial-ankle PWV (cf- and ba-PWV) have been analyzed separately and subgroup analyses by gender are reported. Among 692 potentially relevant works, 24 articles were analyzed.
Results: Seven studies referred to cf-PWV in the general population with an overall positive association at adjusted analysis for both males and females (beta regression coefficient (ß): 0.07; 95%CI: 0.03; 0.11 and ß: 0.06; 95%CI: 0.03; 0.09, respectively). Twelve studies referred to ba-PWV in the general population with the finding of a positive association at adjusted analysis for females (ß: 0.04; 95% confidence interval (CI): 0.01;0.07), but not for males (ß: 0.13; 95%CI: −0.09; 0.34). In hypertensive patients only four studies evaluated cf-PWV and one ba-PWV with only one study (with cf-PWV) finding positive association.
Conclusion: The association between SUA and cf-PWV resulted significant in general population in both males and females while it was only significant for female regarding ba-PWV. Furthermore, the few available studies found no significant relationship between SUA and both cf- and ba-PWV in hypertensive subjects.
Introduction
Serum uric acid (SUA) values have been related to the development of fatal and non-fatal cardio-vascular (CV) events both in hypertensive patients [Citation1] and in the general population [Citation2]. However, the mechanism by which SUA could increase CV events is not fully elucidated. Among various hypothesis, one could be that SUA negatively act at the level of the heart, vessels and kidney determining the so called target organ damage (TOD).
Arterial stiffness (a kind of vascular TOD) is determined by changes in vascular structure and function [Citation3] that reflects the advancement of arteriosclerosis and is an independent predictor of CV and all-cause mortality [Citation4].
The association between SUA and pulse wave velocity (PWV), i.e. the most widely used measurements of arterial stiffness, has been extensively evaluated but some differences in results have been observed [Citation5–Citation28]. Majority of these studies are cross-sectional while only few of them were longitudinal [Citation29–Citation32].
A further important limitation refers to the fact that only few data were analyzed taking into account of the possible effects of gender. In fact, when these analyses were conducted some results were confirmed only in males or in females [Citation23,Citation28,Citation33]. Moreover, SUA and PWV are known to be both higher in males [Citation33], thus denoting a confounding role of sex in the evaluation of this association.
The purpose of this study was to estimate the association between SUA and arterial stiffness in hypertensive patients and in general population, as a whole population and as divided by gender by a systematic review with meta-analysis. Arterial stiffness has been considered when evaluated as PWV. Two different methods are available to measure this CV risk biomarker: carotid-femoral (cf-PWV) and brachial-ankle (ba-PWV). Since the two evaluate different vessels (only elastic one in the first case and elastic and resistive in the second one), they have been analyzed separately.
Material and methods
The meta-analyses followed a study protocol (PROSPERO 2018 CRD42018090397 available from: http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42018090397). Reviewing and reporting were performed according to the recommendations of the Cochrane Collaboration [Citation34] and PRISMA [Citation35], respectively.
Relevant studies were identified by PubMed, Embase, Cochrane and grey literature sources up to October 2018. No languages restrictions were used. The first search from PubMed, Embase, Cochrane and Graylit was performed with the help of a librarian with the following keywords: (Vascular Stiffness OR Vascular Calcification OR Pulse Wave Analysis OR arterial elasticity OR pulse wave velocity) AND (Uric Acid OR Hyperuricaemia OR urate). The full search strategy is shown in Appendix section.
Study selection
After excluding duplicates, citations have been imported in the Covidence software (https://www.covidence.org/) and title and abstract have been screened independently by two researchers (NTand EP). After the first screening, relevant studies were retrieved in full text to check eligibility and specific inclusion/exclusion criteria. A third researcher (AM) reviewed all selections and reconciled disagreement.
We included any observational study dealing with the association between uric acid and PWV in the adult population (age >18). As we already mentioned, we considered separately study measuring cf- and ba-PWV, respectively. In case of multiple publications reporting results from the same dataset, the most updated or comprehensive information was used.
Data extraction and quality assessment
We have developed and tested a sheet for the extraction of the following information from each included study: year of publication; country; study design; inclusion criteria; setting; sample size; mean age; percentages of men and women; unit of measurement and measurement method of SUA levels, measurement method of PWV, effect measure used for PWV, its value and standard deviation (or any other measure of variability). If necessary data were not reported the corresponding author was contacted to obtain this information. If no information was obtained the needed data were obtained by combination or transformation when possible [Citation34].
The risk of bias was assessed by using the Newcastle-Ottawa quality assessment scale for cross sectional, cohort and case control studies (see Supplementary Table 1). The assessment was made independently by two reviewers and possible disagreements were solved by consensus meetings.
Statistical analysis
The standardized regression coefficient, with 95% confidence interval (CI), from multiple linear regression was adopted as the common measure of association across studies. It gives the change in standard deviation units in the average value of pulse wave velocity per standard deviation increase in the uric acid. The standardized coefficient was adopted in order to being able to compare results on cf- and on ba-PWV, respectively. When unstandardized coefficient was reported they were standardized [Citation36]. The summary estimate of the association between uric acid and PWV has been computed by pooling the adjusted correlation coefficients (i.e. the standardized regression coefficients) converted to the Fisher’s z scale [Citation37] across studies. All studies dealing with cf-PWV reported the regression coefficient from multiple linear regression, while five studies measuring ba-PWV reported odd ratio (OR) analyzing UA and PWV as categorical variables. As cut-off used for categorizing PWV were not uniform and UA was categorized in quartiles [Citation5–Citation8] or with a predefined cut-off [Citation9,Citation10] we did not include these studies in the main meta-analysis on ba-PWV, but included them in a secondary analysis, in which the effect size of the association was evaluated in terms of log OR.
We provided the overall estimate and stratified for gender. Given the known confounding effect of gender, we decided to not combine female and male estimates within study (when available) to avoid ecological bias and given that many studies provided only separate estimates, but to include male and female estimates separately in the meta-analysis.
Too few studies on hypertensive patients have been published to be quantitatively meta-analyzed and their results have only been described. As additional analysis we also considered unadjusted Pearson correlation coefficients, however adjusted association estimate was the measure of interest. A minimum set of confounders was specified (age and blood pressure and a further variable) in order to obtain a good score at the Newcastle-Ottawa quality assessment scale and the more complete model was chosen in the presence of more than one multivariable model.
A two-sided test with p value equal or lower than 0.05 was considered statistically significant. Publication bias was assessed by Funnel plots and Egger test. In all cases, a random-effects model was used [Citation37] and heterogeneity of the effect measures was also assessed using the Q and I2 statistics.
Results
Literature search
We identified 692 potentially relevant citations from electronic databases reporting results on the association between SUA and PWV. Of these 603 were excluded after the title and abstract review and 55 as referring to duplicated publication on the same data. After the review of the remaining 34 studies, 10 were excluded for: no baseline data on longitudinal study (1), not respecting inclusion criteria for outcome measure (3), not available on-line also on journal website (3), not respecting inclusion criteria for target population (1) and no data on the association of interest (2). reports the PRISMA flow diagram. Among the 24 selected studies [Citation5–Citation28], 11 referred to cf-PWV, four of which focussed on hypertensive population and 13 to ba-PWV, of which only one focussed on hypertensive population.
Study characteristics
Twenty-four independent samples reporting 67,470 individuals were identified (). Sixteen were conducted in Asian countries and eight in western countries. The selected studies were all cross-sectional, published between 2003 and 2018 and the number of subjects per study ranged between 222 and 12,517.
Table 1. Study characteristics.
reports the risk of bias assessment according with the Newcastle-Ottawa scales. In the selection domain, eight studies resulted of fair quality (two stars) and 16 of good quality (three or four stars). Only two studies justified sample size while five studies poorly described the method used for uric acid measurement. Regarding this, all the studies included in this meta-analysis used the gold-standard enzymatic methods while five of them did not describe reagents or commercial names [Citation7,Citation10,Citation20,Citation24,Citation27]. Four studies resulted of poor quality (zero stars) for the comparability domain, not providing an adjusted association between uric acid and pulse wave velocity and one of fair quality adjusting only for age and blood pressure. Three studies resulted of poor quality and eight studies of fair quality in the outcome domain, mainly due to a poor description and appropriateness of statistical methods and PWV assessment. In fact, for cf-PWV, one study used the PP-1000 pulse wave analyzer (Hanbyul Meditech Co., Jeonju, Korea) [Citation20], one the PulsePen device (DiaTecne srl, Milano, Italy) [Citation24] and one a custom tonometer (Cardiovascular Engineering, Norwood, MA) [Citation27]. Regarding ba-PWV two study doesn’t report the commercial name of the equipment used although they well described the methods [Citation7,Citation10]. Overall, the quality resulted higher for studies measuring cf-PWV, with a total score higher or equal than seven for nine of the 11 studies, with respect studies measuring ba-PWV, in which only six out of 13 studies got a score higher or equal than seven.
Table 2. Quality assessment of included studies according to the Newcastle-Ottawa scales for cross-sectional studies.
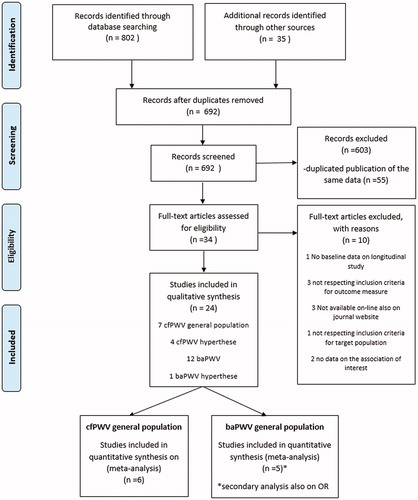
Results are summarized in and and .
Figure 2. Forest plot on standardized regression coefficients on the association between SUA and cf-PWV.
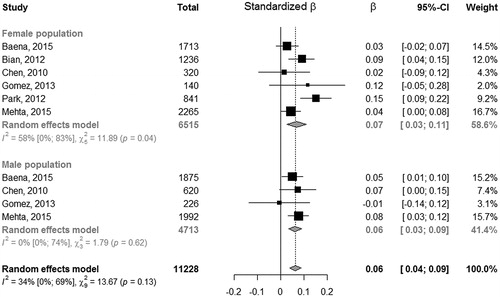
Table 3. Summarizing table for results of meta-analysis of standardized regression coefficients of multivariable linear regression analysis between PWV and UA.
Carotid-femoral PWV and uric acid in the general population
Among the 24 included studies, seven used as outcome cf-PWV and referred to the general population, of which one didn’t provide gender information and was not included in the meta-analysis [Citation24] and one focussed only on females [Citation20]. Thus, six studies focussed on females getting an overall standardized beta (ß) regression coefficient of 0.07 (95% CI: 0.03; 0.11), with estimates of single studies ranging from 0.02 to 0.15. Among the five studies focussing on males, one did not provided the association estimate [Citation26]: the overall standardized ß regression coefficient for males resulted 0.06 (95% CI: 0.03;0.09), with single studies estimates ranging from −0.01 to 0.08. No significant differences between males and females were observed (Q = 0.08, p = 0.77). The overall estimate resulted 0.06 (95%CI: 0.04; 0.09), providing a significant but slight association between PWV and SUA (see ). This means that for each increment of a unit of standard deviation in SUA, PWV increased by 0.06 standard deviations. Overall we observed a low/moderate heterogeneity of the association between SUA and cf-PWV with a proportion of variation in effect size of about 34.2% (95% CI: 0; 69%) of the total observed variance. The Funnel plots (Supplementary Figure 1) did not provide evidence of publication bias (Egger test for Funnel plots asymmetry 0.1108, p = 0.9117).
In appendix Supplementary Figure 2 it is shown the forest plot of the meta-analysis on unadjusted correlation coefficients that also showed a positive correlation between UA and cf-PWV both in males and females, although with a significant difference among sex (Q = 5.14, p = 0.023). The correlation was lower in males (r = 0.10, 95% CI: 0.06;0.14), with respect to females (r = 0.22, 95% CI: 0.12; 0.31), that also showed a high heterogeneity among studies (I2=89%).
Carotid-femoral PWV and uric acid in the hypertensive population
Only four studies dealt with the hyperthensive population [Citation16,Citation17,Citation25,Citation28] and so results have not been meta-analyzed. Three out of four studies didn’t find a significant association on multivariable models between SUA and cf-PWV. Both Tsioufus et al. [Citation16] and Mulè et al. [Citation25] found a significant unadjusted correlation on the entire sample (r = 0.16, p = 0.009 and r = 0.24, p < 0.001, respectively), that lose statistical significance after adjusting for confounders (no estimate provided). Maloberti et al. [Citation28] did not found any association between UA and cf-PWV neither in unadjusted nor in adjusted analyses. On the contrary the study by Vlachopoulos et al. [Citation17] found a positive significant association between UA and cf-PWV also after adjusting for confounders (ß = 0.169) that was also confirmed separately in males and females with a higher Beta in this last group (ß = 0.120 and 0.184, respectively).
Tsioufus et al. [Citation16] and Mulè et al. [Citation25] didn’t provided separate analyses for males and females, while Maloberti et al. [Citation28] didn’t found differences in gender analysis.
Brachial ankle PWV and uric acid in the general population
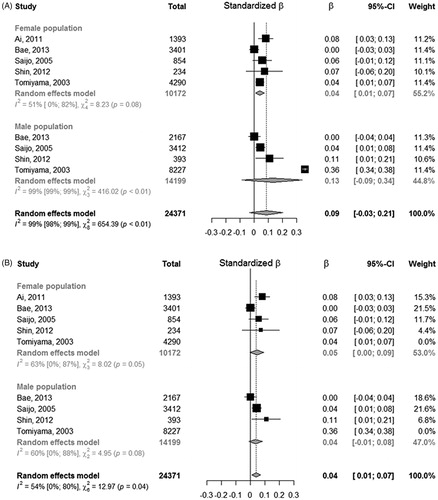
We found 12 studies evaluating the association between ba-PWV and SUA in the general population. Among them, one did not provide an adjusted estimate and was included only in the unadjusted estimation [Citation14] and another one did not provide any analysis by gender [Citation10], but it only showed an OR of 1.001 (95% CI: 1;1.002, p = 0.021) from multivariable logistic regression between UA and PWV (abnormal vs. normal) in the entire population. Five studies fitted a logistic regression after categorizing SUA and ba-PWV and the remaining five studies used a multiple linear regression. Among these the standardized beta regression coefficient of females resulted 0.04 (95% CI: 0.01;0.07), with estimates of single studies ranging from 0.0001 to 0.07. Among males, one study did not report the association estimate [Citation15]: the overall standardized beta regression coefficient for males resulted 0.13 (95% CI: −0.09;0.34), with single studies estimates ranging from 0.0006 to 0.36. This last estimate from Tomiyama et al. [Citation11] was very far from the others and yielded a strong heterogeneity among association estimates. The overall estimate resulted 0.09 (95% CI: −0.03; 0.21), providing a not significant association between ba-PWV and uric acid (, panel A). As noted for males we observed strong heterogeneity of the association between SUA and ba-PWV (I2= 99%). Of note, by removing the study from Tomiyama et al. [11] heterogeneity among studies reduced to an I2=54% and the standardized beta regression coefficient for males changed to 0.04 (95% CI: −0.01; 0.08) remaining not significant (, panel B). Without this study the overall estimated beta regression coefficient resulted significant with an estimate of 0.04 (95% CI: 0.01; 0.07). The Funnel plots (Supplementary Figure 3) did not provided evidence of asymmetry (Egger test: −0.3955, p = 0.6925), however it underlined the presence of big studies with estimates quite far from the overall random effect estimate, proving low confidence on the meta-analysis result.
Figure 3. Forest plot on standardized regression coefficients on the association between SUA and ba-PWV.
As secondary analysis we also considered those studies categorizing UA and ba-PWV: four studies categorized UA in quartiles [Citation5–Citation8] and we decided to show the OR of the forth quartile with respect to the first one, used as reference category by three studies: we excluded from the quantitative analysis the study that used as reference category the second quartile [Citation7]. We also excluded a study that considered two categories for UA using pre-specified cut-off as these were not comparable with the others [Citation9]. We did not found differences between males and females (Q = 0, pvalue = 0.99), the overall OR comparing the forth quartile of SUA with respect to the first one on abnormal ba-PWV resulted 1.70 (95% CI: 1.21;2.40), with a pretty strong heterogeneity among studies (I2=70%, see Supplementary Figure 4).
In appendix Supplementary Figure 5 it is shown the forest plot of the meta-analysis on unadjusted correlation coefficients that showed a positive correlation between SUA and ba-PWV both in males and females, with lower coefficients for males than females, but no evidence of different effect among sex (Q = 2.82, p = 0.0933). The overall estimate of the unadjusted correlation coefficient resulted 0.14 (95% CI: 0.08; 0.20).
Brachial ankle PWV and uric acid in hypertensive population
Only one study focussed on the association between ba-PWV and UA in hypertensive [Citation18]. In this study only very old subjects (aged 80 and over) were evaluated and no significant association were found both in unadjusted and adjusted analyses.
Sensitivity analysis
Sensitivity analysis excluding those studies with a low score for outcome assessment were also done and results are consistent as showed in Supplementary Figure 6. In particular for cf-PWV we got a standardized beta regression coefficient of 0.05 (95% CI: 0.01; 0.10) and 0.05 (95% CI: 0.03; 0.08) for females and for males, respectively. As far as for ba-PWV the studies with a low score for outcome assessment were already excluded for other reasons no further analysis was performed. In fact, Cai et al. [Citation10] did not perform separate analyses for gender and Lee et al. [Citation7] presented results of the logistic regression with a reference category different from the other studies.
Discussion
The results of our meta-analysis seem to demonstrate that SUA is a significant determinant of cf-PWV in general population in both male and female while it is the case only for females for ba-PWV. Furthermore, the few available studies on hypertensives seems not to confirm this relationship (both as cf- and ba-PWV) in those subjects.
There are a variety of mechanisms (either direct or indirect) that could act as a possible link between UA and arterial stiffness. Principally, UA contributes to the arterial stiffening process due to its capacity to determine oxidative stress and due to its pro-inflammatory effects. Furthermore, it activates the local renin-angiotensin system that, together with the two previously cited effects, is able to determine thickening of the vascular wall via promoting proliferation and differentiation of smooth muscle cells and endothelial dysfunction [Citation38]. Finally, hyperuricaemia has been related to a higher prevalence of diabetes mellitus (DM), arterial hypertension and metabolic syndrome [Citation39,Citation40]. Those are important determinants of arterial stiffness and gives us the physio- pathological basis for this relationship.
Regarding gender issue, difference has been found in general population analysis for ba-PWV where results were confirmed in females but not in males. In females SUA levels seems to be more related to glycaemia, insulin resistance and DM and to correlate more with blood pressure levels and MS presence [Citation39,Citation40]. Those are all alterations that could act on the arterial stiffness process helping understand why gender differences have been found. Furthermore, explanation could be also that UA metabolism is genetically controlled and sex differences exist in gene function [Citation41] as well as hormones influences (i.e. menopausal status) [Citation42].
As cited in the introduction, although they are both arterial stiffness measurements, cf- and ba-PWV have been analyzed separately. Although both are well validated [Citation3] and strongly related to CV events [Citation4,Citation43] they are not interchangeable since they represent different measures of arterial stiffness. In fact, the evaluation of ba-PWV include also a large part of medium sized and resistive arteries while in cf-PWV only elastic aorta is assessed. Furthermore, up to now, no data are available on the effects of UA on different types of arteries, so this remain a strong confounding factor.
In the ba-PWV meta-analysis a strong heterogeneity among studies has been observed, in particularly due to the study of Tomiyama et al. [Citation11] that provided a very high estimate that lead to uncertainty that avoided to reach a significant association between UA and ba-PWV. Interestingly, by ignoring this estimate, heterogeneity decreased and UA and ba-PWV resulted to be significantly associated in the overall population and in females. This study resulted of good quality in the domains considered in the Newcaste-Ottawa quality assessment scale, but a typo in reporting the result cannot be excluded. Moreover, a secondary analysis on studies categorizing UA and ba-PWV showed a significant association between UA and ba-PWV.
Instead, different results were observed when we went through hypertensives. In fact, for cf-PWV, three out of four studies did not find a significant association on multivariable model between SUA and cf-PWV [Citation16,Citation25,Citation28]. Only the study by Vlachopoulos et al. [Citation17] found a positive significant association between UA and cf-PWV also after adjusting for confounders. The principal difference between this last study and the others are that in this case only not treated patients have been included in the analysis. This is something very important for the correlation between SUA and PWV. In fact, newly diagnosed untreated patients present, obviously, a shorter history of BP elevation. More than BP values itself also hypertension duration are determinants of PWV [Citation44]. Another point needs to be mentioned, i.e. the effects of antihypertensive therapies on the association between SUA and cf-PWV. In fact, different BP drugs have been found to exert different effects on PWV modification with ACE-I, ARB and b-blockers that strongly act on arterial stiffness [Citation45]. Only one study focussed on hypertensive population reported on drug therapies [Citation28] but it did not include it in the analysis. Therefore, it is possible that longer hypertension history as well as anti-hypertensive therapies could overshadow the effects of SUA on PWV.
Obviously, also a very old age can overshadow the effects of secondary determinants. This is probably the case of the only study that evaluated ba-PWV in hypertensives [Citation18]. In fact, only patients age 80 and over have been evaluated founding that only the age and BP are the main determinants of the arterial stiffness.
Although a publication bias cannot be excluded, given the observational nature of the studies without published protocols the Funnel plots did not provide any evidence of that. Overall the included studies resulted of a fair quality, with the most critical issue in the reporting of statistical analyses, while representativeness, exposure/outcome evaluation and adjustment resulted mainly of good or fair quality.
Similar data can also be obtained from the few longitudinal studies available. Only four longitudinal studies were previously published on this topic [Citation29– Citation32]. Among them, only one focussed on hypertensive patients [Citation32] finding significant association between SUA and cf-PWV progression (i.e. ΔPWV) at univariate analysis (β = 0.15, 95% CI: 0.03;0.27, p = 0.016) that was confirmed only in females. At multivariable analyses the significance was lost in the whole population and in gender analysis also. So, the absence of a strong relationship between SUA and PWV in hypertensives were confirmed in the only available prospective study.
In the general population Canepa et al. [Citation29] found an association between SUA and ΔPWV in men but not in females. Also, in this case significance was lost in the fully corrected multivariable model. Ding et al. [Citation30] found a borderline association between SUA and the presence of arterial stiffness (cf-PWV > 12 m/s) at the follow-up examination (OR 1.824; p = 0.046) but did not give data on the relationship with ΔPWV as well as did not perform gender related analysis. Finally, Nagano et al. [Citation31] found a significant association between SUA and follow-up ba-PWV (β = 0.117; p < 0.05) at multivariable analysis.
In summary, in general population longitudinal studies give heterogeneous results. In fact, one work clearly relates UA with arterial stiffness [Citation31], one did not find this relationship [Citation29] and the latter found only a borderline connection [Citation30]. Heterogeneity could be driven by methodological differences (the first study used ba-PWV while the other two cf-PWV) as well as differences in population selection and follow-up time (ranging from five [Citation29,Citation30] to 10 years [Citation31].
One important limitation of our meta-analysis is the possible influence of factors that are well known determinants of arterial stiffness [Citation45] also on UA levels. As an example, a reduction in kidney function both increases arterial stiffness and UA levels (due to a lower UA excretion) [Citation31]. Thus, even if there is a significant relationship after meta-analysis, this is still only an observation that is not independent of other determinants.
Conclusion
In conclusion, SUA seems to be a significant determinant of cf-PWV in the general population in both males and females while it seems to be significant principally in females for ba-PWV. Furthermore, the few available studies found no significant relationship between SUA and both cf- and ba-PWV in hypertensive subjects. More longitudinal studies are needed in order to definitively clarify the role of SUA in the development and progression of arterial stiffness.
supplemental_figs.zip
Download Zip (734 KB)Supplemental_file_rev.doc
Download MS Word (219 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Gu J, Fan YQ, Zhang HL, et al. Serum uric acid is associated with incidence of heart failure with preserved ejection fraction and cardiovascular events in patients with arterial hypertension. J Clin Hypertens. 2018;20(3):560–567.
- Zhong C, Zhong X, Xu T, et al. Sex-specific relationship between serum uric acid and risk of stroke: a dose-response meta-analysis of prospective studies. J Am Heart Assoc. 2017;6(4):e005042.
- Maloberti A, Vallerio P, Triglione N, et al. Vascular aging and disease of the large vessels: role of inflammation. High Blood Press Cardiovasc Prev. 2019;26(3):175–182.
- Ben-Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63(7):636–646.
- Fang JI, Wu JS, Yang YC, et al. High uric acid level associated with increased arterial stiffness in apparently healthy women. Atherosclerosis. 2014;236(2):389–393.
- Lin Y, Lai X, Chen G, et al. Association among serum uric acid, cardiovascular risk, and arterial stiffness: a cross-sectional study in She ethnic minority group of Fujian Province in China. J Endocrinol Invest. 2012;35(3):290–297.
- Lee H, Jung YH, Kwon YJ, et al. Uric acid level has a J-shaped association with arterial stiffness in Korean postmenopausal women. Korean J Fam Med. 2017;38(6):333–337.
- Ishizaka N, Ishizaka Y, Toda E, et al. Higher serum uric acid is associated with increased arterial stiffness in Japanese individuals. Atherosclerosis. 2007;192(1):131–137.
- Kuo CF, Yu KH, Luo SF, et al. Role of uric acid in the link between arterial stiffness and cardiac hypertrophy: a cross-sectional study. Rheumatology. 2010;49(6):1189–1196.
- Cai XB, Liu YW, Sun FJ, et al. Factors influencing brachial-ankle pulse wave velocity in people undergoing health examination and model evaluation: report of 4 159 case. Acad J Second Mil Med Univ. 2015;36(5):569–572.
- Tomiyama H, Yamashina A, Arai T, et al. Influences of age and gender on results of noninvasive brachial-ankle pulse wave velocity measurement–a survey of 12517 subjects. Atherosclerosis. 2003;166(2):303–309.
- Saijo Y, Utsugi M, Yoshioka E, et al. Relationships of C-reactive protein, uric acid, and glomerular filtration rate to arterial stiffness in Japanese subjects. J Hum Hypertens. 2005;19(11):907–913.
- Chen X, Li Y, Sheng CS, et al. Association of serum uric acid with aortic stiffness and pressure in a Chinese workplace setting. Am J Hypertens. 2010;23(4):387–392.
- Lim HE, Kim SH, Kim EJ, et al. Clinical value of serum uric Acid in patients with suspected coronary artery disease. Korean J Intern Med. 2010;25(1):21–26.
- Ai ZS, Li J, Liu ZM, et al. Reference value of brachial-ankle pulse wave velocity for the eastern Chinese population and potential influencing factors. Braz J Med Biol Res. 2011;44:1000–1005.
- Tsioufis C, Kyvelou S, Dimitriadis K, et al. The diverse associations of uric acid with low-grade inflammation, adiponectin and arterial stiffness in never-treated hypertensives. J Hum Hypertens. 2011;25(9):554–559.
- Vlachopoulos C, Xaplanteris P, Vyssoulis G, et al. Association of serum uric acid level with aortic stiffness and arterial wave reflections in newly diagnosed, never-treated hypertension. Am J Hypertens. 2011;24(1):33–39.
- Bian PD, Pan HH, Li XY, et al. Associated factors of brachial-ankle pulse wave velocity in hypertensive patients aged 80 and over. CNS Neurosci Ther. 2012;18(2):188–190.
- Bian S, Ye P. Gender-specific impact of serum uric acid level on regional arterial stiffness and wave reflection in general chinese population. Heart. 2012;98(Suppl 2):E139–E139.
- Park JS, Kang S, Ahn CW, et al. Relationships between serum uric acid, adiponectin and arterial stiffness in postmenopausal women. Maturitas. 2012;73(4):344–348.
- Shin JY, Lee HR, Shim JY. Significance of high-normal serum uric acid level as a risk factor for arterial stiffness in healthy Korean men. Vasc Med. 2012;17(1):37–43.
- Bae U, Shim JY, Lee HR, et al. Serum carcinoembryonic antigen level is associated with arterial stiffness in healthy Korean adult. Clin Chim Acta. 2013; 415:286–289.
- Gomez-Marcos MA, Recio-Rodriguez JI, Patino-Alonso MC, et al. Relationship between uric acid and vascular structure and function in hypertensive patients and sex-related differences. Am J Hypertens. 2013;26(5):599–607.
- Cicero AF, Salvi P, D’Addato S, et al. Association between serum uric acid, hypertension, vascular stiffness and subclinical atherosclerosis: data from the Brisighella Heart Study. J Hypertens. 2014;32(1):57–64.
- Mulè G, Riccobene R, Castiglia A, et al. Relationships between mild hyperuricaemia and aortic stiffness in untreated hypertensive patients. Nutr Metab Cardiovasc Dis. 2014;24(7):744–750.
- Baena CP, Lotufo PA, Mill JG, et al. Serum uric acid and pulse wave velocity among healthy adults: baseline data from the Brazilian longitudinal study of adult health (ELSA-Brasil). Am J Hypertens. 2015;28(8):966–970.
- Mehta T, Nuccio E, McFann K, et al. Association of uric acid with vascular stiffness in the Framingham heart study. Am J Hypertens. 2015;28(7):877–883.
- Maloberti A, Maggioni S, Occhi L, et al. Sex-related relationships between uric acid and target organ damage in hypertension. J Clin Hypertens. 2018;20(1):193–200.
- Canepa M, Viazzi F, Strait JB, et al. Longitudinal association between serum uric acid and arterial stiffness: results from the Baltimore longitudinal study of aging. Hypertension. 2017;69(2):228–235.
- Ding XH, Wang X, Cao R, et al. A higher baseline plasma uric acid level is an independent predictor of arterial stiffness: a community-based prospective study. Medicine. 2017;96(6):e5957.
- Nagano S, Takahashi M, Miyai N, et al. Association of serum uric acid with subsequent arterial stiffness and renal function in normotensive subjects. Hypertens Res. 2017;40(6):620–624.
- Maloberti A, Rebora P, Andreano A, et al. Pulse wave velocity progression over a medium-term follow-up in hypertensives: focus on uric acid. J Clin Hypertens. 2019;21(7):975–983.
- Redon P, Maloberti A, Facchetti R, et al. Gender-related differences in serum uric acid in treated hypertensive patients from central and east European countries: findings from the blood pressure control rate and cardiovascular risk profile study. J Hypertens. 2019;37(2):380–388.
- Higgins J, Green S. Cochrane handbook for systematic reviews of interventions. Oxford: Wiley-Blackwell; 2008.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100.
- Vittinghoff E. Regression methods in biostatistics linear, logistic, survival, and repeated measures models. Statistics for Biology and Health. 2nd ed. New York: Springer; 2012.
- Borenstein M. Introduction to meta-analysis. Oxford: Wiley; 2009.
- Kruger R, Mothae M, Smith W. Yia 03-07 reactive oxygen species adversely relates to early vascular changes and arterial stiffness in black normotensive smokers: the African-Predict Study. J Hypertens. 2016;34:e205–e206.
- Krzystek-Korpacka M, Patryn E, Kustrzeba-Wojcicka I, et al. Gender-specific association of serum uric acid with metabolic syndrome and its components in juvenile obesity. Clin Chem Lab Med. 2011;49(1):129–136.
- Bombelli M, Quarti-Trevano Tadic M, Facchetti R, et al. Uric acid and risk of new-onset metabolic syndrome, impaired fasting glucose and diabetes mellitus in a general Italian population: data from the Pressioni Arteriose Monitorate E Loro Associazioni study. J Hypertens. 2018;36(7):1492–1498.
- Kolz M, Johnson T, Sanna S, et al. Meta-analysis of 28141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet. 2009;5(6):e1000504.
- Yu S, Yang H, Guo X, et al. Hyperuricemia is independently associated with left ventricular hypertrophy in post-menopausal women but not in pre-menopausal women in rural Northeast China. Gynecol Endocrinol. 2015;31(9):736–741.
- Fortier C, Desjardins MP, Agharazii M. Aortic-brachial pulse wave velocity ratio: a measure of arterial stiffness gradient not affected by mean arterial pressure. Pulse . 2017;5(1-4):117–124.
- Meani P, Maloberti A, Sormani P, et al. Determinants of carotid-femoral pulse wave velocity progression in hypertensive patients over a 3.7 years follow-up. Blood Press. 2018;27(1):32–40.
- Nakamura Y, Fujii S, Hoshino J, et al. Selective angiotensin receptor antagonisms with valsartan decreases arterial stiffness independently of blood pressure lowering in hypertensive patients. Hypertens Res. 2005;28(12):937–943.